- #1
FishmanGeertz
- 190
- 0
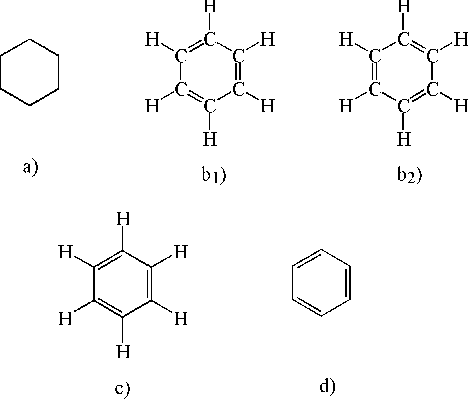
In chemistry and biochemistry, what do the hexagons with letters in between them mean? Call me silly but for some reason they never taught this to us in school.