- #1
yosty222
- 2
- 0
- TL;DR Summary
- Where does the kinetic energy term of the hamiltonian come from? Is it an expectation value of the kinetic energy?
I'm reading through Hohenberg's seminal paper titled: "Inhomogeneous Electron Gas" that help lay the foundation for what we know of as Density Functional Theory (DFT) by proving the existence of a universal functional that exactly matches the ground-state energy of a system with a given interaction potential v(r). I'm working through this paper and I'm a bit confused on the exact form of the Hamiltonian he builds:
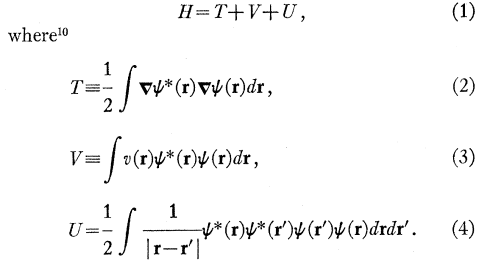
The form of kinetic energy looks like an expectation value, but not quite as I'd expect the expectation value of kinetic energy to look like ## \int \psi^* \nabla^2 \psi ##. The factor of ##\frac{1}{2}## out front makes me think it's coming from a ##\frac{\mathbf{p^2}}{2m}## term (with m = 1 in these units), but why does a ##\nabla## operator get attached to each ##\psi^*## and ##\psi##?
Similarly, for the form of V (equation (3) above), is this expressing the contribution of the external potential ##v(\mathbf{r})## as an expectation value of the external potential over some volume d##\mathbf{r}## then integrating over all space?
The form of kinetic energy looks like an expectation value, but not quite as I'd expect the expectation value of kinetic energy to look like ## \int \psi^* \nabla^2 \psi ##. The factor of ##\frac{1}{2}## out front makes me think it's coming from a ##\frac{\mathbf{p^2}}{2m}## term (with m = 1 in these units), but why does a ##\nabla## operator get attached to each ##\psi^*## and ##\psi##?
Similarly, for the form of V (equation (3) above), is this expressing the contribution of the external potential ##v(\mathbf{r})## as an expectation value of the external potential over some volume d##\mathbf{r}## then integrating over all space?