You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Electron Definition and 999 Threads
The electron is a subatomic particle, symbol e− or β−, whose electric charge is negative one elementary charge. Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure. The electron has a mass that is approximately 1/1836 that of the proton. Quantum mechanical properties of the electron include an intrinsic angular momentum (spin) of a half-integer value, expressed in units of the reduced Planck constant, ħ. Being fermions, no two electrons can occupy the same quantum state, in accordance with the Pauli exclusion principle. Like all elementary particles, electrons exhibit properties of both particles and waves: they can collide with other particles and can be diffracted like light. The wave properties of electrons are easier to observe with experiments than those of other particles like neutrons and protons because electrons have a lower mass and hence a longer de Broglie wavelength for a given energy.
Electrons play an essential role in numerous physical phenomena, such as electricity, magnetism, chemistry and thermal conductivity, and they also participate in gravitational, electromagnetic and weak interactions. Since an electron has charge, it has a surrounding electric field, and if that electron is moving relative to an observer, said observer will observe it to generate a magnetic field. Electromagnetic fields produced from other sources will affect the motion of an electron according to the Lorentz force law. Electrons radiate or absorb energy in the form of photons when they are accelerated. Laboratory instruments are capable of trapping individual electrons as well as electron plasma by the use of electromagnetic fields. Special telescopes can detect electron plasma in outer space. Electrons are involved in many applications such as tribology or frictional charging, electrolysis, electrochemistry, battery technologies, electronics, welding, cathode ray tubes, photoelectricity, photovoltaic solar panels, electron microscopes, radiation therapy, lasers, gaseous ionization detectors and particle accelerators.
Interactions involving electrons with other subatomic particles are of interest in fields such as chemistry and nuclear physics. The Coulomb force interaction between the positive protons within atomic nuclei and the negative electrons without, allows the composition of the two known as atoms. Ionization or differences in the proportions of negative electrons versus positive nuclei changes the binding energy of an atomic system. The exchange or sharing of the electrons between two or more atoms is the main cause of chemical bonding. In 1838, British natural philosopher Richard Laming first hypothesized the concept of an indivisible quantity of electric charge to explain the chemical properties of atoms. Irish physicist George Johnstone Stoney named this charge 'electron' in 1891, and J. J. Thomson and his team of British physicists identified it as a particle in 1897 during the cathode ray tube experiment. Electrons can also participate in nuclear reactions, such as nucleosynthesis in stars, where they are known as beta particles. Electrons can be created through beta decay of radioactive isotopes and in high-energy collisions, for instance when cosmic rays enter the atmosphere. The antiparticle of the electron is called the positron; it is identical to the electron except that it carries electrical charge of the opposite sign. When an electron collides with a positron, both particles can be annihilated, producing gamma ray photons.
View More On Wikipedia.org
Electrons play an essential role in numerous physical phenomena, such as electricity, magnetism, chemistry and thermal conductivity, and they also participate in gravitational, electromagnetic and weak interactions. Since an electron has charge, it has a surrounding electric field, and if that electron is moving relative to an observer, said observer will observe it to generate a magnetic field. Electromagnetic fields produced from other sources will affect the motion of an electron according to the Lorentz force law. Electrons radiate or absorb energy in the form of photons when they are accelerated. Laboratory instruments are capable of trapping individual electrons as well as electron plasma by the use of electromagnetic fields. Special telescopes can detect electron plasma in outer space. Electrons are involved in many applications such as tribology or frictional charging, electrolysis, electrochemistry, battery technologies, electronics, welding, cathode ray tubes, photoelectricity, photovoltaic solar panels, electron microscopes, radiation therapy, lasers, gaseous ionization detectors and particle accelerators.
Interactions involving electrons with other subatomic particles are of interest in fields such as chemistry and nuclear physics. The Coulomb force interaction between the positive protons within atomic nuclei and the negative electrons without, allows the composition of the two known as atoms. Ionization or differences in the proportions of negative electrons versus positive nuclei changes the binding energy of an atomic system. The exchange or sharing of the electrons between two or more atoms is the main cause of chemical bonding. In 1838, British natural philosopher Richard Laming first hypothesized the concept of an indivisible quantity of electric charge to explain the chemical properties of atoms. Irish physicist George Johnstone Stoney named this charge 'electron' in 1891, and J. J. Thomson and his team of British physicists identified it as a particle in 1897 during the cathode ray tube experiment. Electrons can also participate in nuclear reactions, such as nucleosynthesis in stars, where they are known as beta particles. Electrons can be created through beta decay of radioactive isotopes and in high-energy collisions, for instance when cosmic rays enter the atmosphere. The antiparticle of the electron is called the positron; it is identical to the electron except that it carries electrical charge of the opposite sign. When an electron collides with a positron, both particles can be annihilated, producing gamma ray photons.
View More On Wikipedia.org
-
R
I General Magnetic Dipole Moment For an Electron in an Atom
On the first attached page ##\mu_z## is associated with orbital angular momentum (Eq. 41.34). On the following pages (Eq. 41.38) it is associated with spin angular momentum? Are these both part of the same thing? I tried to read further but the book does not address this. In example 41.6 it...- rtareen
- Thread
- Replies: 10
- Forum: Quantum Physics
-
A
Calculating the force on an electron from two positive point charges
So this is more of an intuitive question rather than a mathematical one. I present the problem. Assume I have 2 charges of charge +q at a distance r from each other on the z axis. Position of two charges is (0,0,r/2) and (0,0,-r/2). Assume now that I want to calculate the force these two...- Antonis Hadjipittas
- Thread
- Replies: 2
- Forum: Electromagnetism
-

B Does the energy of an electron vary in the sublevels?
So I read that Bohr's atom has discrete energy levels that an Electron can orbit at and that each level has n amount of sublevels (if n = 2 then there are 2 sublevels). Does the sublevel that the Electron is in have to do with it's mass? Does an electron in energy level l and sublevel d have...- AdvaitDhingra
- Thread
- Replies: 10
- Forum: Quantum Physics
-

I Intuitive/classical picture of electron spin g-factor of 2?
It's been troubling me for a while, is there some kind of intuitive heuristic picture of why the electron spin g-factor is 2? I remembered this question because of the thread about the nature of spin. One of the early models of spin that were proposed was that it represented the electrons...- AndreasC
- Thread
-
- Tags
- Electron Electron spin Picture Spin
- Replies: 5
- Forum: Quantum Physics
-
D
Calculate the Energy Levels of an Electron in a Finite Potential Well
Thank you for reading :bow: Section 1 To find the energy states of the particle, we define the wave function over three discrete domains defined by the sets ##\left\{x<-L\right\}##, ##\left\{-L<x<L\right\}##, and ##\left\{L<x\right\}##. The time independent Schrodinder equation is...- docnet
- Thread
- Replies: 4
- Forum: Advanced Physics Homework Help
-
K
I Electron angular momentum in diatomic molecules
Hello! I just started reading some molecular physics and I am a bit confused about the electron angular momentum in diatomic molecules. Let's say we have just 2 protons and an electron for simplicity and we are in the Born-Oppenheimer approximation, so we assume that the nuclei are fixed in...- kelly0303
- Thread
- Replies: 4
- Forum: Atomic and Condensed Matter
-

Calculating Net Force on an Electron using E(r) and mx
I just insterted E(r) into F to get the net force. After that I wrote mx''=Fnet and I don't know how to proceed (supposing that's the right way).- CBA
- Thread
-
- Tags
- Displacement Electron
- Replies: 9
- Forum: Introductory Physics Homework Help
-
J
I Do electron orbitals ever change?
1. Do electron orbitals ever change in _shape_? Specifically, does a solid have the same orbital shapes as a liquid? 2. Are there any factors that would change the _size_ of electron orbitals?- jactor
- Thread
- Replies: 13
- Forum: Atomic and Condensed Matter
-
J
B Photon detection methods -- do they always involve displacing an electron?
Am I right in thinking that all photon detection methods depend on a photon displacing an electron, that then displaces other electrons to give a detectable electric current pulse?- jeremyfiennes
- Thread
- Replies: 23
- Forum: Quantum Physics
-

The magnetic field just above a lens in an electron microscope
I'm looking for an estimation or simulation of the magnetic field in the horizontal plane just above a typical lens in a transmission electron microscope. A rough cross section of such a lens can be seen here: electron lens - Bing images . The lens is cylindrically symmetric around the vertical...- Philip Koeck
- Thread
- Replies: 1
- Forum: Electromagnetism
-
L
Identical Wires with different voltage--does electron behavior differ?
Homework Statement:: I am studying on my own, so I don't have a specific homework statement, but want to make sure I am thinking about things correctly. What I am wondering is if you have equivalent wires, let's say both are made of copper, and one wire has three times the voltage of the...- LouisL
- Thread
- Replies: 19
- Forum: Electromagnetism
-
E
I Uncertainties of the hadronic corrections for electron and muon
The pie chart in this link shows that about 99.95% of the total error of g-2 of the muon in the theoretical prediction is due to the uncertainties in the hadronic corrections. What is this number for g-2 of the electron? Maybe this number exists also for tau particles? The new value of fine...- exponent137
- Thread
-
- Tags
- Electron Muon Uncertainties
- Replies: 3
- Forum: High Energy, Nuclear, Particle Physics
-

I Semiclassical model of electron dynamics
Why do we exclude interband transitions in semiclassical model of electton dynamics? I mean why it is an assumption in this model?- Rzbs
- Thread
- Replies: 6
- Forum: Atomic and Condensed Matter
-

Find the position of the electron at any time t
At present i am only attempting the part a, i want to use the equation ##F=ma ; qE = ma;## ---> eq1 The electric field is given by the formula ##E = -\frac {dV} {dx} ## ##v = \frac {dx} {dt} => dx = v_0 {dt}## ---> eq2 (?) ##E = -\frac{dV} {v_0 dt}## Here ##V = -V_m \sin(\omega t) ## hence...- PhysicsTest
- Thread
- Replies: 7
- Forum: Introductory Physics Homework Help
-
H
B Is an electron beam affected by photons?
I am wondering if one of the prerequisites of the double-slit experiment, when done with electrons, is that the beams must be in a dark vacuum tube so as to not destroy the interference pattern. I am trying to learn if the beams will lose their interference pattern because the particles of the...- Herbascious J
- Thread
-
- Tags
- Beam Electron Electron beam Photons
- Replies: 4
- Forum: Quantum Physics
-
J
Electron moles present in wire cross-section
So I know current is just coulombs/second. Electrons are also in the unit of coulombs, so I can get coulombs to cancel. 7.9C/s/1.602E-29C = 4.93133E29 1/s Now I just need to get mol on top. There are 6.022E23 electron in a mol so 4.93133E29 1/s / 6.022E23 atoms/mol = 8.1889E5 mol/s. Now my...- JoeyBob
- Thread
- Replies: 7
- Forum: Introductory Physics Homework Help
-

Photoemission from a non-interacting electron gas
I had another excercise of the long list of the same topic (solid state physic) where I need a bit of help. All other excercise where about interband transition, dispersion relation, refracting and absorption coefficient, x-rays and so on, and I managed to solve them or I think I will be able...- SQUB
- Thread
-
- Tags
- Electron Electron gas Gas
- Replies: 1
- Forum: Advanced Physics Homework Help
-
T
B Pair Instability Supernovae & Electron Capture Supernovae
Hi! I've been browsing the internet for information about supernovae and I came across this chart describing 4 types of core collapse causes (the chart may have copied weirdly because not all the information fits into this text box): Cause of collapse Progenitor star approximate initial mass...- tovisonnenberg
- Thread
- Replies: 2
- Forum: Astronomy and Astrophysics
-

I Ontology of an electron passing through a Stern-Gerlach magnet
So, as far as I think I understand, an electron that passes through a Stern-Gerlach magnet, will not have a value for its spin until that spin is measured? Does that mean the electron has no position (as given by the SGM) until measured, or that the electron does not even exist until measured?- entropy1
- Thread
- Replies: 32
- Forum: Quantum Interpretations and Foundations
-

At least One Faraday Tube Between Every Two Unlike Charges in the Universe
By Classic Coulomb's Law there exists negligible yet non zero force of attraction between two unlike charges in-spite of the distance. However for electrostatic attraction to work we need at-least one Faraday Tube(Lines of Forces) between the attracting charges, does that means...- Narayanan KR
- Thread
- Replies: 5
- Forum: Electromagnetism
-

Magnetic field lines around electron and wire seem to contradict
In the picture below, the direction of the magnetic field lines can be determined by using the right-hand rule with the thumb pointing in the direction of the current. If we use the right hand rule in the picture below, thinking of the yellow arrow as the current, we would not get the correct...- SamRoss
- Thread
- Replies: 6
- Forum: Electromagnetism
-
I
Explaining Differences in Terbium & Uranium Electron Configs
Why is terbium's electron configuration [Xe] 6s2 4f9, with no electrons in the d subshell and one extra in the f sub shell, while uranium's electron configuration is [Rn] 7s2 5f3 6d1, with one electron in the d subshell and none extra in the f subshell? Are lanthanum and actinide d block...- i_love_science
- Thread
- Replies: 3
- Forum: Chemistry
-
S
Compton Effect with the electron initially moving
Hello! I do not understand how to prove the exercise. I have searched all over but I have found no hints on how to get started. Can anybody help me?- sciencec
- Thread
- Replies: 13
- Forum: Introductory Physics Homework Help
-
S
B Question about energy needed by electron to change energy levels
Electron can move from lower energy level to higher energy level when it absorbs energy equal to the difference between the energy levels based on equation: ##hf = \Delta E## If the incident photon has lower energy compared to ##\Delta E##, then electron won't move to higher energy level. But...- songoku
- Thread
- Replies: 6
- Forum: Quantum Physics
-
F
B Why doesn't the electron "wave" collapse at the double-slit?
This is dumb question, so please bear with me. In the double-slit experiment where they fire a single electron at time, as you can see the electron gun fires a single electron. Now the electron travels as a wave. Now my question is, why doesn't the wave collapse when the wave encounters...- francis20520
- Thread
-
- Tags
- Collapse Double-slit Electron Wave
- Replies: 28
- Forum: Quantum Physics
-

I Electron correlation vs electron exchange
What is the difference between electron correlation and electron exchange? Which of them is due to the spin of electrons and which is due to charge of electrons?- Rzbs
- Thread
-
- Tags
- Correlation Electron Exchange
- Replies: 4
- Forum: Atomic and Condensed Matter
-
J
I Is the electric field of an atom a superposition or mean of electron positions?
We usually think about atomic orbital as wave(function), but it was created from e.g. electron and proton approaching ~10^-10m (or much more for Rydberg atoms), and electron has associated electric field. This wavefunction also describes probability distribution for finding electron (confirmed...- Jarek 31
- Thread
- Replies: 18
- Forum: Quantum Interpretations and Foundations
-

I Exploring Dynamics of Electron in Hydrogen Atom
Hello,everyone. Can dynamical system be used to describe the behavior of the electron in Hydrogen atom?- thaiqi
- Thread
- Replies: 7
- Forum: Quantum Physics
-
A
How Can the Optimum Aperture Angle in Electron Beam Lithography Be Determined?
Hi, Is there anybody who knows about this subject and can guide kindly? Regards, In electron beam lithography, there is an optimum aperture angle to obtain a minimum beam size: a) Determine this angle considering only the influence of the source and spherical aberrations. b) What is the...- anni
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-

B Dual slit with controlled electron trajectories
In the traditional single electron duel slit experiment, I assume a cathode emits electrons in an unfocused direction spreading across the dual slits like a flashlight beam, but one electron at a time. Electrons however can be finely focused and controlled using magnetic or electric fields...- Buckethead
- Thread
-
- Tags
- Dual Electron Slit Trajectories
- Replies: 26
- Forum: Quantum Physics
-

MHB Coulomb's Constant in electron energy formula.
Hi, If we multiply $En=-\frac{2\pi^2me^4Z^2}{ n^2h^2} $by $\frac{1}{(4\pi\epsilon_0)^2},$ it is the formula of electron energy in nth Bohr’s orbit. Why we should multiply it by $\frac{1}{ (4\pi\epsilon_0)^2}$ a Coulomb's constant in electrostatic force? Where m=mass of electron, e= charge...- WMDhamnekar
- Thread
- Replies: 1
- Forum: General Math
-

Exploring Electron Gun Deflection: Causes and Resources for Understanding
Hi, I was wondering if anybody knows why the plate in an electron gun is tilted with respect to the electron path? Or has any resources that I could read to better understand? Thanks.- SWKatzen
- Thread
- Replies: 7
- Forum: Electromagnetism
-
M
Electron between two parallel plates
a) E = s / E0 so s is 4.87E-9 b) The electron will be projected at up angle since its charge is negative ( not sure if there's another reason behind it) c) Initial speed: V0 = 5 * 10^6 * cos(theta) + 5 * 10^6 * sin(theta)The force suffered by the electron is: Fy = q*Ey Fy = -1.602*10^19 *...- Mike94
- Thread
- Replies: 6
- Forum: Introductory Physics Homework Help
-

Schrodinger equation in three dimensions. Atom with one electron.
When solving the Schrodinger equation by separation of variables to atom with one electron and in the spherical coordinates, we get $$\Psi = \Theta(\theta)\phi(\varphi)R(r)$$ Specifically, $$\phi = e^{im\rho }$$ The question is, why we adopt this particular solution, in general, we have this...- LCSphysicist
- Thread
- Replies: 2
- Forum: Advanced Physics Homework Help
-
C
Exploring Electrons: A Mind-Blowing Video Explanation
I stumbled across this on youtube and wondered if someone could explain what we are actually looking at here. Looks amazing!- cmb
- Thread
- Replies: 2
- Forum: Electromagnetism
-

I Loss of electron & proton energy due to radiation
Can you compare the energy loss of electrons and protons due to the radiation they emit? In fact, I want to know which of the two loses more energy when it emits radiation.- Adams2020
- Thread
- Replies: 9
- Forum: High Energy, Nuclear, Particle Physics
-

How Do Electron and Proton Accelerations Compare Between Charged Plates?
Ve=0m/s Vp= 0m/s Qe/Qp= 1.60E-19 Me=9.11E-31 Mp-1.67E-27 Ive pretty much gathered all of the equations I think I need to solve the problem. I just am stuck. The last step I realize that the forces would be equal to each other so I have mp x ap = me x ae but then when I try to solve for the...- Sj4600
- Thread
- Replies: 7
- Forum: Introductory Physics Homework Help
-
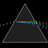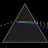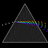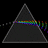
Hydrogen Emission Spectrum and Electron Energy Levels
1. The 4th line from the left, being the aqua blue line, corresponds to a wavelength of 486 nm, as blue light has a wavelength in the range 450-495 nm. 2. This is where I am having the most difficulty, I have tried to answer the question comprehensively but I am not satisfied with my answer. In...- AN630078
- Thread
- Replies: 2
- Forum: Introductory Physics Homework Help
-
R
Electron moving to the face of plate from depth d
I don't really understand the question. I don't understand the wording. We know the plate has a thickness L = 0.50cm. If the charge is coming from the battery wouldn't the electrons have to move the entire distance to reach the face of the plate? Because they have to move all the way from the...- rtareen
- Thread
- Replies: 4
- Forum: Introductory Physics Homework Help
-

Relaxation time and average electron velocity in Drude model
If τ is the relaxation time, τ means, on average the time between two collisions for an electron moving under a constant electric field inside a metal. Now according to the assumptions of drude model, the electron acquires an additional velocity of \frac{-eEt}{m}where t is the time elapsed since...- Sunny Singh
- Thread
- Replies: 1
- Forum: Electromagnetism
-
L
Maximum velocity of an electron emitted from lanthanum
Mass of an electron = 9.1*10^-31 Well, to find the maximum kinetic energy of the electron use E=hf, E=hc/λ=6.63*10^-34*3*10^8/1.5 *10^-7 E=1.326 *10^-18 J 1/2m v^2 max=E Rearrange in terms of v: v=√2E/m v=√2*1.326 *10^-18/ *9.1*10^-31 v=1707127... ~ 171000 ms^-1 Where have I gone wrong here?- lpettigrew
- Thread
- Replies: 11
- Forum: Introductory Physics Homework Help
-
L
B Does an electron have kinetic energy when attached to a proton?
does an electron have kinetic energy when attached to a proton? if not, what is it transformed into?- Lolicon
- Thread
- Replies: 8
- Forum: Quantum Physics
-

Electromagnetic mass of an electron
Hi, I was reading the following Wikipedia article and couldn't make sense of few points. I'd appreciate it if you could help me with it. Source: https://en.wikipedia.org/wiki/Electromagnetic_mass#Rest_mass_and_energy Question 1: What is this "electrostatic energy ##E_{em}##"? Is it some kind...- PainterGuy
- Thread
-
- Tags
- Electromagnetic Electron Mass
- Replies: 3
- Forum: Electromagnetism
-

B How does the electric field of an electron compare to its probability wave?
A single electron sitting in a void has an electric field that spreads out evenly in all directions as far as there is open empty space to allow it, is this roughly a correct statement? Let's say we now introduce a singe proton into the void, 100 miles from the electron - it will also have an...- DarkMattrHole
- Thread
- Replies: 5
- Forum: Quantum Physics
-

I Electron scattering in the Brillouin zone boundary
I what to know what is electron scattering in Brillouin zone boundary? What exactly happen for electron in Brillouin zone boundary; what happen for it in real space and what happen for it in reciprocal space? And is electron scattering from a Brillouin zone boundary could be a source for...- Rzbs
- Thread
- Replies: 4
- Forum: Atomic and Condensed Matter
-

I Can we imagine that an electron moves on an orbit of chaos?
Bohr's hydrogen atom model is outdated facing Schrodinger's wave equation. Now that wave mechanics doesn't use a concept of orbit for the electron in hydrogen atom. But can we suppose the electron is still circling around the atom core, not necessarily in circles or ellipses, but in chaos like a...- thaiqi
- Thread
- Replies: 27
- Forum: Quantum Interpretations and Foundations
-

B Randomness in electron position
So from what I understand the position of an electron at any given time is based on a probability model. My question is how does gravity play a role in this model? Can we say the position of the electron is truly random? What if this "randomness" is caused gravitational forces pulling it in...- kolleamm
- Thread
-
- Tags
- Electron Position Randomness
- Replies: 12
- Forum: Beyond the Standard Models
-

Why not detect and identify SARS-CoV2 using Electron microscopy?
We know the RT-PCR test method currently employed to detect SARS-CoV2 viruses from the sample is not 100% foolproof in detection. If the current electron microscopy can reach a resolution of up to 50pm, why not use this time-tested technology in the detection of this virus? Are there any...- mktsgm
- Thread
- Replies: 4
- Forum: Biology and Medical
-
C
Imaging Emitted Covid 19 Samples with an Electron Microscope
After many months what comes out of the mouth and nose of someone infected with Covid 19 has not been directly observed. A single virus particle of Covid 19 is about 120 nanometers in diameter. This means that to see the virus, visible light wavelengths of 400 nm to 700 nm are too long to...- Chas Tennis
- Thread
- Replies: 25
- Forum: Electromagnetism