- #1
sanman
- 745
- 24
Graphyne is a variant of graphene which has Carbon-Carbon triple-bonds:
http://physics.aps.org/articles/v5/24
Image:
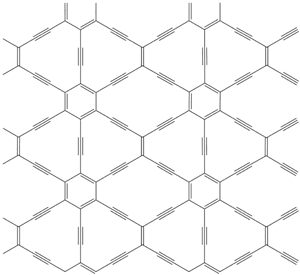
I'm thinking that it wouldn't have superior mechanical properties, since the presence of the triple bond would give a polar character that weakens the SP2-hybridization. However that same polar character might have useful benefits for electron mobility and bandgap properties.
Opinions?
http://physics.aps.org/articles/v5/24
Image:
I'm thinking that it wouldn't have superior mechanical properties, since the presence of the triple bond would give a polar character that weakens the SP2-hybridization. However that same polar character might have useful benefits for electron mobility and bandgap properties.
Opinions?
Last edited: