- #1
aub
- 21
- 0
i know this doesn't fit here right but i didnt know where else i could ask this
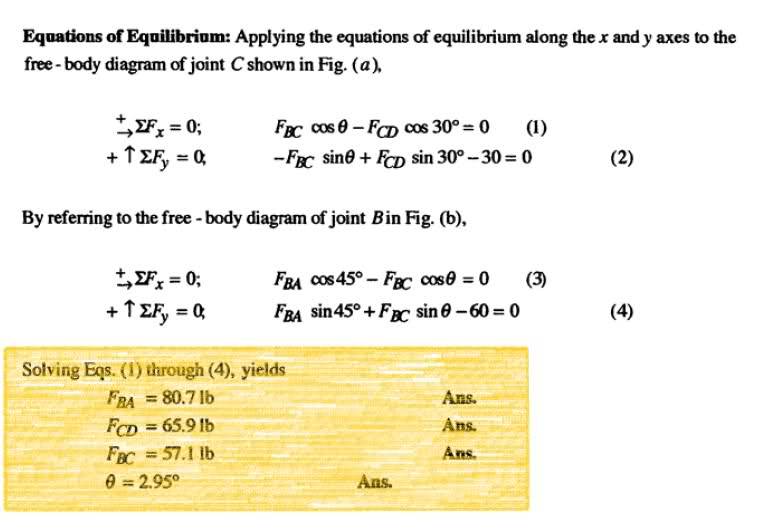
how did they solve 1 through 4? it should be easy but i can't solve it
thanks for any help
Homework Statement
how did they solve 1 through 4? it should be easy but i can't solve it
thanks for any help