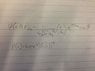- #1
Verruto
- 2
- 0
Homework Statement
Hydrogen is in n=2, l=1, and m=0.
Wave function is ψ(r,θ,∅)=(1/4(√2pi)ab3/2)(r/ab)(e-r/2ab)(cos(θ)
Find <r> for this state.
Homework Equations
P(r) = 4pir2|R(r)|2
<r> is equal to the integral from 0 to ∞ of P(r)dr
The Attempt at a Solution
I understand that you need to go from the wave function to R(r) and then P(r) to put that in the <r> equation. I am just not sure how you do the first step.
I put the equations in the picture below to better visualize it. Thanks in advance!