- #1
manal950
- 177
- 0
Hi
How are you
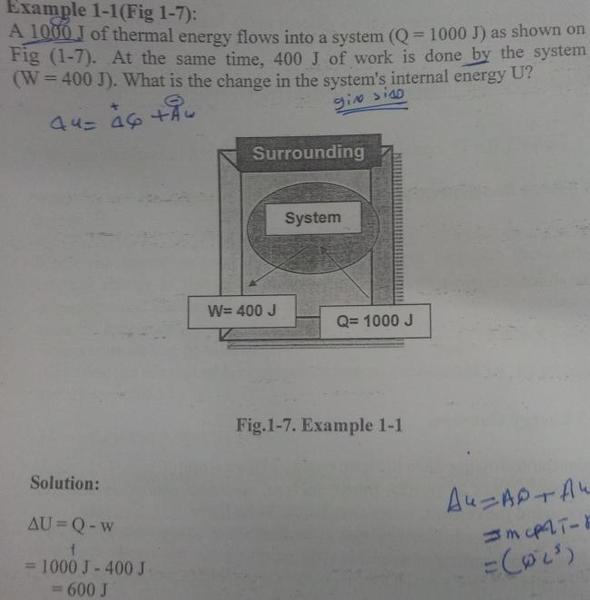
I have a question how we can know the work if - or +
also heat energy
please could see this question why in answer
take work as -
and heat as +
How are you
I have a question how we can know the work if - or +
also heat energy
please could see this question why in answer
take work as -
and heat as +