Endothermic Definition and 55 Threads
-
P
Why Isn't My Endothermic Crystallization Working?
I'm using a process that uses heat to catalyze crystallization. I don't have to equipment to really understand what's going on, it could just be incomplete nucleation from a super saturated solution, but when I cool it, it doesn't crystallize. Now, I am in an acid solution (pH between 4-5) so if...- paradoxlost
- Thread
- Endothermic Heat
- Replies: 6
- Forum: Chemistry
-
S
Mechanical guy needs help with endothermic reaction and heat decomposition
How did you find PF?: Google I am working on a project that requires us to protect components from high heat. We were thinking on using sodium bicarbonate, but our module needs to be exposed FIRST to 85C for long periods.... then the high heat exposure will be present. We believe this...- sanchel
- Thread
- Endothermic Mechanical Reaction
- Replies: 8
- Forum: Mechanical Engineering
-

Would this reaction be exothermic or endothermic?
Summary:: A tertiary allylic alcohol rearranges to become a primary allylic alcohol, would it be exothermic because the final product is more stable and lower in energy? Hi all, this problem's been on my mind for a couple of days now and I'm not making any progress with it. My problem is that...- Beowulf_8991
- Thread
- Endothermic Exothermic Reaction
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-

Safe Endothermic reactions for skin
Hi, Its my first time here in PF, I was wondering what could be a fast endothermic reaction(with water) that does not burn the skin/ cause severe allergic reactions when/after touching or in contact with the reaction. (With high rate of cooling and hopefully no bubbles or gas forming). E.g to be... -
N
Catabolism exergonic yet breaking a bond is endothermic?
Hi - really basic question here, but I was wondering. In biology it's said that catabolic reactions which break something down release energy, making such exergonic. In chemistry it is said that breaking bonds is endo-thermic. This seems counter-intuitive to me? But, I realize it's because the... -
A
Does anyone know of a thermally-stable endothermic reaction?
Hello all, I've used physicsforums a few times over the years, but I believe this is my first post. I'm working on an engineering project in which I need to find a highly-endothermic reaction where the reactants and products will be stable at very high temperatures (well over 800C), or at least...- alphacat25
- Thread
- Chemical Endothermic Fire Reaction
- Replies: 9
- Forum: Chemistry
-
K
Can a reaction be neither exo- nor endothermic?
Homework Statement Organic life is based on complex organic molecules formed from smaller ones during a long evolution. Using data in Appendix 2, investigate one such reaction: 2 C2H6 (g) → C4H10 (g) + H2 (g) (a) Calculate ∆rH and ∆rS for such a reaction under standard conditions (Po = 1 bar...- kingkong23
- Thread
- Endothermic Reaction
- Replies: 11
- Forum: Biology and Chemistry Homework Help
-

Why electron affinity of noble gas is endothermic?
Homework Statement Why is the EA of Neon endothermic even though it has a high Z eff? Basically, what makes a full valence shell so stable? The attempt at a solution I know it has to do with shielding, core e-, and valence e-. But I don't know how to word it.- NuclearBoofluff
- Thread
- Electron Electron affinity Endothermic Gas Stable
- Replies: 3
- Forum: Introductory Physics Homework Help
-
M
Endothermic and Threshold reactions - Are they Equivalent?
Hi, up to this day I thought that endothermic and threshold reactions are equivalent. I mean each endothermic reaction must be threshold and each threshold reactions must be endothermic. But I think I was wrong. Here is example (from this source Q-value): 10B(n,2*alpha)T This threshold...- Michal Kovac
- Thread
- Endothermic Equivalent Reactions Threshold
- Replies: 3
- Forum: Nuclear Engineering
-
N
Why is This Reaction Endothermic?
Homework Statement At 1500 ° C , CO is formed more than at 1000 ° C. Is the reaction from left to right exothermic or endothermic? Homework Equations C(s) + H2O ↔CO(g) + H2[/B]The Attempt at a Solution I think its Exothermic reaction . The temperature is increased from 1000°C to 1500 °C . If...- Nanu Nana
- Thread
- Endothermic Reaction
- Replies: 5
- Forum: Biology and Chemistry Homework Help
-
H
Reversible endothermic reaction
hi, In the past there were "toys" containing a fluid and a clip. When pressing the clip, the fluid became hard and it gave warmth. When the toy was boiled in water, it became fluid again and one could start over. Now I was wondering of there is a chemical reaction that gives cold instead of... -
T
I How endothermic reactions happen?
Ok, this is a large qiestion. Firstly, from second law of thermodynamics, thermal flow always happens between matters kept in different temparature. But while an endothermic reaction takes place, it may extract some heat from environment and consume it. Isnt it against second law? And for...- Tahmeed
- Thread
- Endothermic Exothermic Reactions Thermochemistry
- Replies: 1
- Forum: Classical Physics
-
M
Endergonic reaction vs endothermic reaction.
Homework Statement Are endergonic reactions and endothermic reactions the same thing? Homework Equations N/A The Attempt at a Solution N/A- MBBphys
- Thread
- Endothermic Reaction
- Replies: 3
- Forum: Biology and Chemistry Homework Help
-

Dry ice and acetone, endothermic?
For decades I've wonder. Is there an endothermic reaction between dry ice and acetone? With ice, salt forces a phase change lowering the temperature but that doesn't seem applicable here. -

Tortoises "hibernate", but a tortoise isn't endothermic
In http://en.wikipedia.org/wiki/Hibernation, "hibernation" is defined as being restricted to endotherms. But lots of sites, such as http://www.anapsid.org/hibernation.html, claim that ectotherms can also hibernate: for example, tortoises. First: which definition is correct? If Wikipedia's...- nomadreid
- Thread
- Endothermic
- Replies: 3
- Forum: Biology and Medical
-
A
What is the strongest endothermic reaction involving salt and liquid water?
Hi, I'm looking for the reaction that produces the largest temperature drop/absorbs the most energy from its surroundings. It needs to be some kind of dissolution process of a salt being mixed with liquid water. I've looked around on the internet, and it seems tricky to search for, and I don't...- Alistair1992
- Thread
- Endothermic Reactions
- Replies: 5
- Forum: Chemistry
-

Can a hydraulic compression create endothermic phenomena?
The title might be a bit confusing but I just want to know if you can recreate (partial) the system of refrigeration with hydraulic compression. So say, you have a container (cylindrical piston) filled with water, then you apply pressure. Will the water's temp increase? Then, will the...- deuel18
- Thread
- Compression Endothermic Hydraulic Phenomena
- Replies: 6
- Forum: Mechanical Engineering
-
T
Power generation through endothermic heat absorption.
I have been toying with an idea that may break the second law of thermodynamics for a while now, but it is basically this; An endothermic reaction is used to convert heat energy into chemical energy, and then the products of that reaction are used as the reactants in an electrochemical reaction...- Teslafly
- Thread
- Absorption Endothermic Generation Heat Power Power generation
- Replies: 6
- Forum: Thermodynamics
-
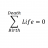
Why can substances with endothermic heat of solution dissolve?
Why is a substance with an endothermic heat of solution able to dissolve? Is it due to the activation energies being reached (forming activated complexes)? If so then I'm guessing this has to do with temperature.- STEMucator
- Thread
- Endothermic
- Replies: 1
- Forum: Chemistry
-
E
Entropy and Endothermic Processes
I am a general chemistry student and I find thermodynamics fascinating. However, I have a hard time visualizing entropy. Can somebody please explain how an increase in entropy can make a process that is endothermic spontaneous? The typical demonstration of entropy that I have seen is on in... -
Q
Is Reaction A Exothermic in Synthesis of Water?
Homework Statement http://i4.minus.com/j7HwKoL8yhl96.JPG Homework Equations The enthalpy is equal to the heat of the products minus the heat of the reactants. The Attempt at a Solution We are not supposed to use a bond enthalpy table to ascertain the enthalpy of the reactions...- Qube
- Thread
- Endothermic Reactions
- Replies: 8
- Forum: Introductory Physics Homework Help
-
A
Striking a match, Endothermic or Exothermic?
Homework Statement I was wondering whether striking a match is endothermic or exothermic. Homework EquationsThe Attempt at a Solution This seems quite straight forward as striking a match releases heat, which is obviously exothermic. However, the process of igniting the match requires heat...- Acnhduy
- Thread
- Endothermic Exothermic Match
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
C
Why is ionization of sodium endothermic?
Homework Statement State whether ionization is an endothermic or an exothermic process. The Attempt at a Solution I know what exothermic and endothermic mean, and I know that the answer is that ionization of sodium is an endothermic process, but I don't know why and I'm hoping someone can...- CatWhisperer
- Thread
- Endothermic Ionization Sodium
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-
D
How Much Heat Is Involved if 927 Grams of Mg(OH)2 Reacts?
Homework Statement given: 2 H3PO4 + 3 Mg(OH)2 + 1427 kj → 6 H20 + Mg3(PO4)2 How much heat is involved if 927 grams of Mg(OH)2 reacts? Homework Equations The Attempt at a Solution 927 g x Mg(OH)2 mole/56.305g x 1427 kj/3Mg(OH)2 mole = 70,482 kj I just felt like I...- davie08
- Thread
- Endothermic Heat Reaction
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
T
Determine if chemical equation is exothermic or endothermic?
How would I determine, if given a chemical equation is exothermic or endothermic? Eg. Mg + 2HCL\rightarrowMgCl2 + H2- TheRedDevil18
- Thread
- Chemical Endothermic Exothermic
- Replies: 2
- Forum: Chemistry
-
C
Endothermic Reactions: Activation Energy & Heat Transfer
every reaction requires its reactants to have enough actvation energy in order to start the chemical reaction.sometimes, extra energy is provided in the form of heat. so the reactants take in heat from the surroundings and have enough energy to break old bonds and form new bonds. when they break... -
1
Practical Endothermic reactions
I understand that spontaneous endothermic reactions are used rigorously in the design of instant cold packs to treat injures. Can someone please list and suggest other uses of spontanous endothermic reactions that can be used practically in everyday life -
A
Energy of products in endothermic reaction
How is the energy of products in endothermic reaction more than the energy of reactants When we see the following equation of an endothermic reaction below 2H2O2--> 2H2O + O2 The 2H2O2 has an enthalpy of 2144 whereas the products have and enthalpy of 1424 Kj/mol SO the reactants have more... -
I
Can someone explain why this dissolution is exothermic rather than endothermic?
A pair of students found the temperature of 100 g of water to be 27.0°C. They then dissolved 6.32 g of KOH in the water. When the salt had dissolved, the temperature of the water was 42.5°C. If the temperature of the water is raised, doesn't that mean it retained heat, thus showing that the...- IntegrateMe
- Thread
- Endothermic Exothermic Explain
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
H
Endothermic absorb energy How do they begin in the first place?
Endothermic absorb energy... How do they begin in the first place?? PLEASE, BEFORE ANSWERING I would like you to hear me out. >>> Im really confused as to the phenomenon of why endothermic reactions between mixing of two substances just spontaneously happen.. giving them temporary "cold"...- hondaman520
- Thread
- Endothermic Energy
- Replies: 2
- Forum: Chemistry
-
C
Looking for a safe endothermic chemical reaction
I’m not very well versed in chemistry so am asking for help. I’m looking for a couple of safe chemicals which combine to react (or dissolve etc.) endothermically. It doesn’t have to be an impressive reaction, it just has to be safe and not produce masses of gas (so that’s carbonate/ bicarbonate... -
D
Is this nuclear reaction exothermic or endothermic?
Is this nuclear reaction exothermic or endothermic?? Homework Statement Is this nuclear reaction exothermic or endothermic?? alpha particle + beryllium -> carbon12 + neutron Homework Equations The Attempt at a Solution Im not quiet sure which one it is, I don't know what...- dav1d
- Thread
- Endothermic Exothermic Nuclear Nuclear reaction Reaction
- Replies: 2
- Forum: Introductory Physics Homework Help
-
G
Is this homework assignment exothermic or endothermic?
Homework Statement Label each of the following reactions as exothermic or endothermic ("exo" or "endo"), and according to whether work is done on or by the system, or no work is done at all ("on", "by" or "none")? Note that no "endo-on" cases appear here, as these are always thermodynamically...- gurtaj
- Thread
- Endothermic Exothermic
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
S
Endothermic vs Exothermic Reactions in Ammonia Production
Homework Statement Is the reaction for the production of ammonia an endothermic reaction or an exothermic reaction? Explain. Homework Equations n/a The Attempt at a Solution I know that the production of ammonia in the Haber process is indeed exothermic and that the backwards reaction (NH3...- Spacec0wboy
- Thread
- Ammonia Endothermic Exothermic Reactions
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
H
Endothermic / exothermic reaction
Hello, I need a few compounds that i can use that are soluble with water that causes the water to cool, so that if ingested it will not do any harm. your advice would be greatly appreciated, also if you may include a chemical formula if it is known. THANK YOU. P.S. this is out of interest... -
Q
Creating an Endothermic Reaction Without Ammonium Nitrate
So, I'm building a freeze ray, in a sense, and I was wondering... What would the easiest/cheapest way to produce an endothermic reaction without using ammonium nitrate? The way I have the schematics so far is that I will have two substances that will blend just before exiting the ray, and... -
C
Endothermic reaction: where has the energy disapeared?
Assume a thermodynamic system S entirely isolated from the rest of the world, consisting in a block of salt inside in a tube containing water at some initial temperature. The total calorific energy of S is E. Now, after a time T, the bloc of salt has dissolved itself completely or partially in...- coquelicot
- Thread
- Endothermic Energy Reaction
- Replies: 11
- Forum: Classical Physics
-
S
Using Endothermic Solvation To Assist Distillation
From time to time I find myself dissolving potassium nitrate in water (endothermic solvation) and a while back i considered that if i was boil a saturated solution of potassium nitrate that it would be easier than boiling distilled water; as evaporation would have to be exothermic, which would... -
D
Thermodynamics: Exothermic & endothermic reaction in counter current flow
Hi guys, I have a scenario here; let's say we have a exothermic reaction and a endothermic reaction going in co-current flow reactor (separated by a thin wall to facilitate heat transfer). At steady state after some time, from the concentration profile, we have the endothermic reaction going at...- de85
- Thread
- Counter Current Current flow Endothermic Exothermic Flow Reaction Thermodynamics
- Replies: 1
- Forum: Electromagnetism
-
Q
Binding energy and endothermic reactions
from masteringphyics.com, ||\\\\\\\\ the binding energy of a nucleus is defined as the difference between the rest energy that the individual particles would have if they were not bound in a nucleus and the rest energy of the nucleus itself. So, stated another way, fusion reactions are...- quietrain
- Thread
- Binding energy Endothermic Energy Reactions
- Replies: 3
- Forum: Classical Physics
-

Is Hell exothermic or endothermic?
Let's assume for the sake of discussion that hell is not "neither".- Char. Limit
- Thread
- Endothermic Exothermic
- Replies: 8
- Forum: General Discussion
-
K
Are Endothermic Nuclear Reactions Possible in Nature?
Nuclear reactions in which the Q value is negative (endothermic reactions) imply that energy is absorbed in the process of the reaction. Is this to be interpreted as a case where the initial rest mass energy is less than the rest mass energy of the products of the reaction? If affirmative, it...- kihr
- Thread
- Endothermic Nuclear Reactions
- Replies: 10
- Forum: High Energy, Nuclear, Particle Physics
-
D
Endothermic Paint: Absorbing Heat and Keeping Cool
Ok so there's this paint that has been produced which contains small glass beads. When painted on to a wall it acts as insulation. Very good insulation in fact. I was listening to a radio show where a person was talking about it and he described that it was endothermic in nature. Now i know what...- dnorric
- Thread
- Endothermic Paint
- Replies: 3
- Forum: Other Physics Topics
-
D
How is ionization an endothermic reaction?
I don't get how ionization is an endothermic reaction. In an endothermic Reactions: the reactants have less potential energy than do the products. Energy must be input in order to raise the particles up to the higher energy level. The ionization energy, is the energy required to completely...- dolimitless
- Thread
- Endothermic Ionization Reaction
- Replies: 2
- Forum: Other Physics Topics
-
D
Hi. a simple question on endothermic fusion
Would it be feasible theoretically to use a continuous endothermic fusion reaction as a high-energy heat sink? Would such a process be capable of producing unstable isotopes for use in nuclear fission? I ask this as a science fiction writer and I would dearly love input and even direction or...- duration
- Thread
- Endothermic Fusion Hi
- Replies: 4
- Forum: High Energy, Nuclear, Particle Physics
-
I
Is hell exothermic or endothermic interesting find
Apparently students were given an assignment to prove whether hell obeyed the laws of thermodynamics and if it was endothermic or exothermic. The most interesting response is given below. http://wfhummel.cnchost.com/hell.html author unkown.- Ian_Brooks
- Thread
- Endothermic Exothermic Interesting
- Replies: 7
- Forum: General Discussion
-
P
Spontaneous endothermic nuclear reaction?
is such a thing possible? I am curious to know if this could/does exist. An alternative would be if someone gave an example of a reaction where electron capture occurs and the product ends up being stable enough to not decay into anything further. Thanks (btw, does cold fission exist...- pancake
- Thread
- Endothermic Nuclear Nuclear reaction Reaction Spontaneous
- Replies: 9
- Forum: High Energy, Nuclear, Particle Physics
-
S
Is Hell exothermic or Endothermic ?
Is Hell exothermic or Endothermic ?? :smile: The following is an actual question given on a University of Washington engineering mid-term. The answer was so "profound" that the Professor shared it with colleagues, and the sharing obviously hasn't ceased... Bonus Question: Is Hell exothermic...- smarter than u
- Thread
- Endothermic Exothermic
- Replies: 8
- Forum: General Discussion
-
W
Is energy transfer in a chemical reaction always from potential to kinetic?
Endothermic / Exothermic I have to say that I am a little at loss here. I understand that an endothermic process absorbs more energy than it releases, and that an exothermic process is the opposite. However, I don't understand the implications in terms of kinetic and potential energy. Here is...- Werg22
- Thread
- Endothermic Exothermic
- Replies: 5
- Forum: Biology and Chemistry Homework Help
-
S
Endothermic Decomposition Experiment
I am doing an assignment and I'm not sure if the answer is endothermic decomposition someone please help me! During an experiment, a metal and an excess of fluorine gas were placed into a combustion chamber at a temperature of 1800°C. The temperature in the sealed chamber continued to rise to...- spicegirl
- Thread
- Decomposition Endothermic Experiment
- Replies: 3
- Forum: Biology and Chemistry Homework Help