Ice melting Definition and 22 Threads
-
R
Problem about a block of ice melting (specific latent heat)
Energy lost by water = Energy gained by ice Energy lost by water = 0.16 x 4200 x (100-t) Energy gained by ice = 0.205 x L + 0.205 x (t) (where t is the temperature at thermal equilibrium). However, there does not appear to be enough info to continue. The solution, however, considered t to be...- RateOfReturn
- Thread
- Block Heat Ice Ice melting Latent heat Melting Physics Thermodynamics
- Replies: 1
- Forum: Introductory Physics Homework Help
-

The Mystery of Heat Loss: Examining Ice Melting Without Heat Loss
During the procedure, 30% of heat is lost. So that means that 70% of water+container is contributing to melting the ice, right? And the other 30% contributing melting the ice is down to, well, the "heat being lost to the surroundings" (not sure what this really means). We compute the sum and...- DarkEnergy890
- Thread
- Heat Heat loss Ice Ice melting Loss Melting Mystery
- Replies: 11
- Forum: Introductory Physics Homework Help
-
C
Comparing Water and Ice Melting for fighting fires
Good morning, I'm wondering on this question for a while. Imagine we have a fire progressing in a corridor 30 meters wide at a known velocity. What kind of barrier (of the following) can be most successful in minimizing or even erasing this fire and why: a water-soaked area, or a zone covered... -
C
Global Ice melt and heat balance
Can anyone point to a refereed paper exploring the heat absorbed by melting around 500 GT of ie per year (Antarctic - 127 GT pa; Greenland 286 GT pa; sea ice, glaciers estim. 100 GT pa)? As latent heat of fusion is 333.55 KJoules per Kg, I reckon the heat absorbed per year is around 1.67 X 1023...- charles65
- Thread
- Balance Global Heat Heat balance Ice Ice melting
- Replies: 5
- Forum: Earth Sciences
-
C
Thermodynamics Ice Melting Question
Homework Statement On a hot summer day, you planned a trip to a beach, but you inadvertently took a wrong turn and now you’re worried the ice in your cooler is going to melt. The cooler is 0.5 m × 0.5 m × 0.4 m, and is made with 5 cm thick Styrofoam (k = 0.033 W/m2K). Help your panicking...- ConnorM
- Thread
- Ice Ice melting Melting Thermodynamics
- Replies: 4
- Forum: Introductory Physics Homework Help
-

Drawing a heat curve of ice melting
Homework Statement Homework Equations equation with each part. The Attempt at a Solution I did part a) b) and c) on my own: Part d: I am having trouble at this part. I understand how to draw the heat curve but I am confused on what numbers I should use for the x-axis of the graph. I...- Evangeline101
- Thread
- Curve Drawing Heat Ice Ice melting Melting
- Replies: 4
- Forum: Introductory Physics Homework Help
-
R
Does the shape of a container affect the heat required to melt ice inside it?
Hello everyone! I recently saw a problem about some ice in 2 containers. So: We have 2 vertical cylindrical containers, which have perfect insulating walls, one with surface of the base S and the other one 2S , filled with the same mass of ice. The question is if there is any diffrence between...- RingNebula57
- Thread
- Cylindrical Heat Ice Ice melting Melting
- Replies: 2
- Forum: Classical Physics
-
I
Is there an electromagnetic transducer to melt ice?
Hi I have a project to were I would like to melt ice from several feet away. Is there an electromagnetic transducer that I can operate to melt ice? What resonate frequency would I need to use? Any ideas? Thanks- itzkovitch
- Thread
- Electromagnetic Electromagnetic induction Electronics Ice Ice melting Transducer
- Replies: 3
- Forum: Electromagnetism
-
M
Calculating Iceberg Melting Rate
So I'm trying to model the process of towing an iceberg from Antarctica to elsewhere to use for fresh water. I hit a dead end trying to find how much of the iceberg would be left. I know it takes 333 J/g to melt ice and the mass of the iceberg is 7 million tons so i know how much energy it...- marawan
- Thread
- Ice melting Melting Rate
- Replies: 2
- Forum: Other Physics Topics
-
P
Total entropy change for ice melting in a room
Homework Statement Okay, so I am having difficulties with understanding the concepts around entropy, take this question: What is the total entropy change for 7 kg of ice melting from -5 C° to 5 C° in room at 5 C°. Homework Equations dS=dQ/T Q =mΔH m*c*ln(tfinal/tinitial) c_ice=2 c_water=4...- plpg
- Thread
- Change Entropy Ice Ice melting Melting
- Replies: 4
- Forum: Introductory Physics Homework Help
-
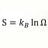
Heat Transfer Coefficient for Ice Melting Time
Homework Statement One liter of water at 30 C ( 30000 calories ) 100 gram (100 cc ) sphere of ice at 0 C is in center of water volume. The ice will absorb 8000 cal melting and final water temp = 22000 cal/ 1100 g = 20 C Assuming mixing and uniform water temp during melting ,and vessel is...- morrobay
- Thread
- Coefficient Heat Heat transfer Heat transfer coefficient Ice Ice melting Melting Time
- Replies: 1
- Forum: Advanced Physics Homework Help
-
M
Solution to Thermo Ice Melting Problem
Homework Statement An insulated beaker with negligible mass contains liquid water with a mass of 0.325 \rm{kg} and a temperature of 69.9 C. How much ice at a temperature of -15.0 C must be dropped into the water so that the final temperature of the system will be 39.0 C? Take the specific heat...- mysticjbyrd
- Thread
- Ice Ice melting Melting Thermo
- Replies: 2
- Forum: Introductory Physics Homework Help
-
D
Ice melting in water in a gravity free fall
Here is the original question: - Well, the answer that I came up with is, that, since there is no eternal force on the system, the centre of mass cannot be displaced, no matter what. Now, my doubts are regarding this itself. When the ice melts, it turns into water of lesser volume. In the...- dharavsolanki
- Thread
- Fall Free fall Gravity Ice Ice melting Melting Water
- Replies: 20
- Forum: Mechanics
-
S
Calculating Enthalpy Change of Ice Melting in Water
If I have a piece of ice melting in a water and eventually comes to equilibrium with the water... The the total enthalpy change includes the following right? Heat absorbed for ice to get to 0 degrees Heat absorbed for ice to melt to water Heat absorbed for the melted ice water to come to...- shredder666
- Thread
- Ice Ice melting Melting Water
- Replies: 1
- Forum: Mechanics
-
J
Fluid mechanics - ice melting into water
Homework Statement A pure ice cube sits in water, floating. Prove that the water level will not change after the ice cube melts (meaning, the volume displaced by the cube will equal the volume of water added once the cube is melted). density of water = 1000 kg / m^3 density of ice = 920...- JRPK8@mizzou.
- Thread
- Fluid Fluid mechanics Ice Ice melting Mechanics Melting Water
- Replies: 1
- Forum: Introductory Physics Homework Help
-
G
Ice melting in water over time?
Homework Statement Hi, I need some help with a homework assignment I have, the scenario is as follows: The Saudi Arabian government has decicded to look into towing a large iceberg from Antarctica to solve problems with low water supply. As their top physics advisor, you are to explore the...- gillwoman
- Thread
- Ice Ice melting Melting Time Water
- Replies: 6
- Forum: Introductory Physics Homework Help
-
T
Thermodynamics and ice melting.
[SOLVED] Thermodynamics and ice melting. Homework Statement An open container holds ice of mass 0.585 kg at a temperature of -13.3 *C. The mass of the container can be ignored. Heat is supplied to the container at the constant rate of 800 J/min. The specific heat of ice to is 2100 J/Kgand...- Terp
- Thread
- Ice Ice melting Melting Thermodynamics
- Replies: 2
- Forum: Introductory Physics Homework Help
-
D
Investigating How Water Volume Affects Ice Melting Rate
Aim: How does the volume of water at a given temperature affect the amount of ice that can melt in it? I did a lab experiment. I put ice in different volumes of water and waited for 5minutes. I measured the amount of ice melted and the final temperature of water. RESULT: (starting...- dumb__x
- Thread
- Ice Ice melting Melting Rate Volume Water
- Replies: 4
- Forum: Introductory Physics Homework Help
-
M
Equilibrium Temperature of Cup and Water When Adding Ice - Homework Solution
Homework Statement A 35 g ice cube at 0.0°C is added to 110 g of water in a 62 g iron cup. The cup and the water have an initial temperature of 36°C. Find the equilibrium temperature of the cup and its contents. Homework Equations heat lost by water+cup...- map7s
- Thread
- Ice Ice melting Melting Water
- Replies: 1
- Forum: Introductory Physics Homework Help
-
S
Solving the Ice Melting Problem - Sergio's Calculation
I have done some calculations so far but i am kind of stuck, here is the problem: A jar of tea is placed in sunlight until it reaches an equilibrium temp of 32.4 deg C. In an attempt to cool the liquid, which has a mass of 185g, 113g of ice at 0degC is added. Assume specific heat capacity of...- S_fabris
- Thread
- Ice Ice melting Melting
- Replies: 6
- Forum: Introductory Physics Homework Help
-
M
Melting Ice: A Comparison of Air and Water Environments
Hello I was wondering: in which environment ice will be melt faster, air or water? I ahd argue with my friend recently, we needed Ice and he put it in pot with moderately cold water. Since we were outside and temperature was normal, about 17-18 Cel degree I said it will melt faster in water...- Micko
- Thread
- Air Ice Ice melting Melting Water
- Replies: 7
- Forum: Other Physics Topics
-
N
Ice melting because of a candle burning?
What law or physics principle is that? Thanks for the help.- Nikola Tesla
- Thread
- Ice Ice melting Melting
- Replies: 4
- Forum: Introductory Physics Homework Help