Molarity Definition and 115 Threads
-

How to predict the volume of a solution?
I've never had a good understanding of this. Say I am dissolving 20g sodium chloride into 1L of water. Is the only way to theoretically predict the final volume of solution to consult density tables? edit: I realize this isn't a practical situation, and that typically you are making a solution... -

Chemistry Calculation of molar conductance at infinite dilution
The answer given is 524*10^4. The process used is by adding/subtracting the values given. But my concern is that isn't molar conductivity an intensive property? And it hence can't be added. So how is it added and the answer obtained.- Zayan
- Thread
- Conductance Dilution Molarity
- Replies: 8
- Forum: Biology and Chemistry Homework Help
-

Calculating Molarity of KOH Solution: Discrepancy in Reported Results
I'm trying to verify the final molarity of my solution. I'm adding 40.69 lb of 90% anhydrous KOH to 148.43 (561.86L) gallons of water . I get a final molarity of 0.52. The report I'm reading arrives at a molarity of 0.78. Is this an error? Thanks to anyone who can provide the calcs. -
S
Molarity is represented by M and its unit is also M?
In my textbook it is given that Molarity is represented by M, and its formula is-- Molarity(M)= No. of moles of solute/Volume of solution in L the unit of Molarity is mol/l which is also written as M. Now what does this mean ? does it mean --> Molarity is denoted by "M" and it's unit is also...- SHASHWAT PRATAP SING
- Thread
- Molarity Unit
- Replies: 23
- Forum: Chemistry
-
J
What Is the Molarity of Potassium Ions in a Potassium Sulfite Solution?
Hi, I have a problem which I have attempted to solve several times without success and i am at a loss to know where I am going wrong. The problem is .. If 10.0 g of potassium sulfite is dissolved in water to make 200.0 mL of solution, then what is the molar concentration of potassium ions in...- jackthehat
- Thread
- Ions Molarity
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-

Is molarity the same as probability?
In the keq derivation intuition video mr sal Khan relates molarity to probability and it doesn't makes sense to me as molarity can also be more than 1 and probability cannot. Can you please tell me how he relates probability to molarity. Thanks -
L
Basic molarity and dilution formulas
ok, for the life of me i can not remember how to do the BASIC math for my issue. I mean, i can do calc but i have forgotten basic math haha. here is my question. If i have 91% grain alcohol, how do i figure out how much water to add to it to drop the percentage to 70%. Dimensional analysis is... -

Chemistry Is the N Factor of Oxalic Acid in Titration Correctly Applied?
I got a answer by equation the moles i took the n factor of oxalic acid as 2 and the naoh solution came out to be 2M but i got an answer of 4grams but the answer given is 2 grams am i correct ??- Prabs3257
- Thread
- Equilibrium Ionic Molarity Moles Titration
- Replies: 12
- Forum: Biology and Chemistry Homework Help
-
J
Difference between diluting and adding
Homework Statement Place 20.0 g NaOH(s) in a flask and dilute to 1.00 L with water. Place 20.0 g NaOH(s) in a flask and add 1.00 L of water. How exactly do these two statements differ? Wouldn't adding 1.00L of water to 20.0g of NaOH dilute it anyways? Homework Equations none? The Attempt...- JessicaHelena
- Thread
- Concentration Difference Dilution Molarity
- Replies: 8
- Forum: Biology and Chemistry Homework Help
-

How can I prepare a 0.1M NaOAc buffer at pH 5 using titration?
For a lab I need to create a 0.1M solution of NaOAc buffer at pH 5 It's been a while since I worked with buffers. I'm struggling to find a way to get both the proper morality and proper pH. My first though was to create a 0.2M solution of acetic acid and titrate it to pH 5 using sodium...- ReidMerrill
- Thread
- Buffer Molarity Ph
- Replies: 4
- Forum: Chemistry
-
A problem in volumetric calculations in Chemistry
Homework Statement :[/B] The density of a 2.05 molar solution of acetic acid is 1.02g/ml. The Molality of the solution is: (a) 1.14 mole/kg (b) 3.28 mole/kg (c) 2.28 mole/g (d) 0.44 mole/kg Homework Equations :[/B] The Attempt at a Solution :[/B] I tried it out very simply using unitary...- Wrichik Basu
- Thread
- Calculations Chemistry Molality Molarity Mole
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
Z
What are the units for Ksp and why are they not always included in calculations?
Homework Statement I have found, through experimentation, the ksp of calcium hydroxide to be 3.0 x10^-7 (which i know has a pretty large percent error). However, i am conflicted as to what the units should be because logically, plugging the units into the ksp equation should give you units of...- Zoey Brown
- Thread
- Ksp Molarity Units
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
A
Molarity vs. Concentration: What's the Difference?
Concentration measures the number of moles of solute per liter of solution, besides that it can be used to measures also the ratio of mass per liter. Molarity also measures the number of mole of a solute per liter of solution. Then what is the difference between these two definitions? They seem... -
T
Solution molarity change in electrolytic cell
Homework Statement The diagram below shows an electrolysis cell which contains 1L of an aqueous 1M copper(II)sulfate solution. If 0.4 moles of electrons pass through the cell, the concentration of copper ions after passage of the charge will be A) 0.4 M B) 0.8 M C) 1.0 M D) 1.2M...- TT0
- Thread
- Cell Change Molarity
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
A
Question about acids And molarity vs concentration
so is an acid like HCl, is it basically these HCl ions that are "dissolved" or "infused" into water and form an acid? So what I'm saying is HCl by itself isn't possible and it's only possible in the dissolved form or aqueas form. This would make sense because as far as I know an acid or base... -
D
Find ph based on given volume and molarity of HCl and NaOH
Hello! I am trying to crack the following problem: What is the pH of a solution formed by mixing 125.0 mL of 0.0250 M HCl with 75.0 mL of 0.0500 M NaOH? Here is how I am approaching the issue: both HCl and NaOH are strong acid and base respectively. Hence the concentration of [H3O+] =...- ducmod
- Thread
- Hcl Molarity Ph Volume
- Replies: 22
- Forum: Biology and Chemistry Homework Help
-
A
Calculating the molarity when mixing two different solutions
Homework Statement You mix 45-mL of 0.1174M K2SO4 and 35-mL of 0.2504M HNO3 Homework Equations none. The Attempt at a Solution So I'm supposed to find the number of moles of the K2SO4 and add it to the number of moles of 0.2504, then add the two volumes, and divide the total number of moles...- AMan24
- Thread
- Mixing Molarity
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-

Spectator Ions and Molarity Questions
Homework Statement An aqueous solution of silver nitrate, AgNO3, reacts with an aqueous solution ammonium carbonate, (NH4)2CO3. What are the spectator ions for the reaction? AgNO3 (aq) + (NH4)2CO3 -> A 17.5 mL sample of hydrochloric acid HCl solution required 29.6 mL of 0.250M Ba(OH)2 for...- B3NR4Y
- Thread
- Ions Molarity
- Replies: 22
- Forum: Biology and Chemistry Homework Help
-

Help w/ Molarity Question Related to a CuSO4 Solution
We have a question on my daughter's homework I'm trying to help with and there is a part of it we have not been able to find support for to get to the answer. See below. We believe we've been able to answer parts "a" and "b" correctly, however, we're having trouble answering part "c" and were...- ptownbro
- Thread
- Molarity Stoichiometry
- Replies: 8
- Forum: Biology and Chemistry Homework Help
-
H
Density of Solution and mass percent
There are two numericals, 1. Density of Methanol is 0.793 kg/l. Find molarity. ANSWER is : Desity/ Molar mass = Molarity (UNDERSTANDABLE) In other numerical 2. Concentration of H2SO4 is 98% by mass having density of 1.84gm/ml. Calculate Molarity. here they used another formula. now my...- harman90
- Thread
- Density Mass Molarity Percent
- Replies: 17
- Forum: Biology and Chemistry Homework Help
-

What is the mass of NH3 per cc in a solution of ammonia gas passed into water?
Ammonia gas is passed into water, yielding a solution of density 0.93 g/cm^3 and containing 18.6% NH3 by weight. The mass of NH3 per cc of the solution is 1) 0.17 2) 0.51 3) 0.34 4) 0.68I solved it using the molarity equation. Molarity = 10xd/MM where, x - % by weight d - density MM -...- Prashasti
- Thread
- Molarity Stoichiometry
- Replies: 7
- Forum: Biology and Chemistry Homework Help
-

What does molarity mean in case of weak electrolytes?
I am really confused about it.Suppose we add 5 moles of acetic acid in 1 litre of water..The molarity of solution will be -(5 moles)/volume of solution?Or it will be -(the number of moles of acetic acid dissociated/dissolved)/volume of solution -
A
Find the Molarity of a Solution Using Concentrated Ammonia (28% by Mass)
Homework Statement Calculate the molarity of a solution made by adding 35.5 mL of concentrated ammonia (28.0 % by mass, density 0.880 g/mL) to some water in a volumetric flask, then adding water to the mark to make exactly 250 mL of solution. (It is important to add concentrated acid or base...- antonisz
- Thread
- Analytical chemistry Chemistry Molarity Stoichiometry
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-

Henderson Hasselbalch Buffers and Volumes
Homework Statement You have 725 mL of an 0.55 acetic acid solution. What volume (V) of 1.30 M NaOH solution must you add in order to prepare an acetate buffer of pH = 4.99? ( The pKa of acetic acid is 4.76.) [/B] Homework Equations pH = pKa + log A/HA Ratio of A- to HA = 10^(pH-pKA)[/B]The...- Meadow_Lark
- Thread
- Acids Buffers Molarity Volumes
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-

Calculating Volume of 0.835M Na2SO4 Solution (0.0534 mol Na+)
Question: What volume of a 0.835 M solution of Na2SO4 do you have if there are 0.0534 mol of Na+ present in the solution? What I did: Since we have 0.0534 moles of Na+ ions in the Na2SO4 molecule, I used that for the equation. Since 0.0534 moles is in a 1:1 ratio with Na2SO4 I can conclude we...- RJLiberator
- Thread
- Molarity Volume
- Replies: 3
- Forum: Biology and Chemistry Homework Help
-
Q
Calculating Molarity and Molality: Tips and Tricks | Chemistry Homework Help
Homework Statement Screenshot of some prof's website. This is just ridiculous. http://i.minus.com/jnUa604Tz6bKX.png Homework Equations Molarity to molality. Molality is moles / kg of solvent. Molarity is moles / volume of solution. The Attempt at a Solution Pretty sure all the ones...- Qube
- Thread
- Molality Molarity
- Replies: 3
- Forum: Biology and Chemistry Homework Help
-
Q
Calculating Mole Fraction from Molality: Sodium Nitrate Solution Example
Homework Statement The molality of an aqueous sodium nitrate solution is 2.14 m. What is the mole fraction of sodium nitrate? Homework Equations Molality = moles of solute / kg of solvent. Mole fraction of A = moles of A / total number of moles (in this case, moles of solute +...- Qube
- Thread
- Molality Molarity
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
K
Chemistry Calculate Mole Fraction, Density, & %w/w given Molarity and Molality?
Homework Statement Consider the following aqueous solution at 25°C Cr2(SO4)3, 1.26 mol/L, 1.37 mol/kg Calculate the mole fraction, density and % w/w Homework Equations χ = n Cr2(SO4)3 / n Cr2(SO4)3 + n H2O Unit conversions ρ= g solvent / mL solution % w/w = mass solvent / 100 g...- kariibex
- Thread
- Density Fraction Molality Molarity Mole Mole fraction
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-

Including water in molar mass for molarity?
Hi, let's say I need 60 mL of a 1M solution of potassium sodium tartrate, I am looking for how many grams to measure out of my stock. So I need to convert mol to grams using molar mass. Do I use the full formula weight that includes water KNaC4H4O6*4H2O, or simply the KNaC4H4O6? -
A
How Do You Calculate the Molarity of Glacial Acetic Acid?
Homework Statement Glacial acetic acid has a density of 1.05g/ml and contains 99.51%acetic acid (CH3COOH) by mass. Calculate the molarity of glacial acetic acid. So, this is a question in my pre-lab that doesn't even count. But, I still want to get it because we have similar questions in...- alingy1
- Thread
- Molarity
- Replies: 5
- Forum: Biology and Chemistry Homework Help
-
P
How Do You Prepare a 13.3 mM Trypan Blue Solution in 30% Ethanol?
Concentrations and Molarity?? Homework Statement This problem was given in class; the teacher left it to us to solve it on our own. He wrote this problem himself: you have 150 millimolar of Trypan Blue. you need to get 13.3 millimolar Trypan Blue in a 30% ethanol solution in EXACTLY 10...- plutolover
- Thread
- Molarity
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-

Estimate molarity from enthelpy, gibbs energy and entropy of formation
Hi there, I have to solve this problem: Use the following data to estimate the molarity of a saturated aqueous solution of ##Sr(IO_3)_2## So, I think I should use the Van't Hoff equation in some way, but I don't know how. I also have: ##\Delta_r G=\Delta G^o+RT\ln K## ##K## is the...- Telemachus
- Thread
- Energy Entropy Estimate Formation Gibbs Molarity
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-

Chemical equilibrium, getting molarity given pH and Kb
What should be the molarity in an aquatic dissolution for Trimethylamine, ##(CH_3)_3N## if ##pH=11,2##? ##(CH_3)_3N+H_2O \rightleftharpoons (CH_3)_3NH^++OH^-##, ##K_b=6.3 \times 10^{-5}## I know that ##pH+pOH=14 \rightarrow pOH=2.8##, then ##[OH^-]=1.58 \times 10^{-3}## And...- Telemachus
- Thread
- Chemical Chemical equilibrium Equilibrium Molarity Ph
- Replies: 15
- Forum: Biology and Chemistry Homework Help
-
Q
How do you calculate moles and neutralization in a buffer solution?
Homework Statement this is a part of a buffer solutions problem 3.3g of solid NaC2H3O2*3H2O added to 46mL of deionized water and 4.0 mL of 6.0M HC2H3O2. a. How many moles of HC2H3O2 are contained in a 19 mL of the above buffer? b. How many moles of NaOH would need to be added to the 19...- qpham26
- Thread
- Buffer Molarity
- Replies: 3
- Forum: Biology and Chemistry Homework Help
-
P
What Steps Solve a Complex Molarity Problem?
Homework Statement http://i.imgur.com/Rz0qB.png Homework Equations The answer is B, but I would like to know how to find the answer. Molarity = moles * volume The Attempt at a Solution Yep, I tried for 15+ minutes and couldn't get the darn units to cancel out. A worked out...- pasido
- Thread
- Complex Molarity
- Replies: 3
- Forum: Biology and Chemistry Homework Help
-
C
Calculate the molarity of a NaOH solution
Homework Statement A 3.125 g sample of primary standard of Na2CO3 was treated with 40.00 mL of dilute perchloric acid. The solution was boiled to remove CO2, following which the excess HClO4 was back-titrated with 10.12 mL of dilute NaOH. In a separate experiment, it was established that...- casey619
- Thread
- Molarity
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
A
Chem EDTA and Molarity Question. Need help
[b]1. We had a titration lab where we titrated tap water with EDTA. Through our results we should be able to figure out the molarity of Ca and Mg ions. I think I'm doing this right but it seems to easy in comparison with everything else we are doing in lab and lecture so I just want to know if...- AMSAMS
- Thread
- Chem Edta Molarity
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
G
Calculating Molarity & Volume of Cu(NO3)2
Homework Statement My problem is in the attachment. I am trying to find the molarity and volume of Cu(NO3)2 while I only know Cu has .50 g and HNO3 is 10M and is 5mL. Homework Equations The Attempt at a Solution I have done my calculations on the right. What I have circled is...- gurpalc
- Thread
- Molarity Volume
- Replies: 5
- Forum: Biology and Chemistry Homework Help
-
A
How Do You Calculate Grams of Solute for Specific Solutions?
Homework Statement Calculate how many grams of solute are necessary to prepare: 1) 100 mL of 0.1 N (Normal Solution) Ca(OH)2 (molecular weight = 74) 2) 100 mL 5% NaCl (molecular weight = 40) Homework Equations Molarity = # of moles / # of liters The Attempt at a Solution 1. I...- azncocoluver
- Thread
- Molarity
- Replies: 5
- Forum: Biology and Chemistry Homework Help
-
A
What Is the Molarity of 70% TBHP Solution?
Homework Statement What is the Molarity of 70% TBHP? Homework Equations Given: d=.937g/ml, FW=90.12g , w/w%? The Attempt at a Solution 1000mL x (0.937g/mL) x (1mol/90.12g) = 10.4M That is what I got but idk what to do with the 70% or that w/w% or is that right the way I did it?- amorale
- Thread
- Molarity
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-
B
Find molarity, seemingly simple but I can't do it.
Homework Statement Lauryl alcohol, C12H25OH, is prepared from coconut oil; it is used to make sodium lauryl sulfate, a synthetic detergent. What is the molarity of lauryl alcohol in a solution of 17.1g lauryl alcohol dissolved in 148g ethanol, C2H5OH? a) 0.310m b) 0.620m c) 0.842m d)...- bublik13
- Thread
- Molarity
- Replies: 3
- Forum: Biology and Chemistry Homework Help
-
I
What is the mass of a liter of solution?
A solution of KCl has a density of 1.011 g/mL and has a concentration of 0.273 M. What is the molality of the solution? The molar mass of KCl is 74.5513 g. So first I got g of KCl solute: .273 (m) * 74.5513 (g/m) = 20.35 g Total mass of solution = 1020.35g (since 1000g solvent per .273m...- IntegrateMe
- Thread
- Molarity
- Replies: 8
- Forum: Biology and Chemistry Homework Help
-
D
Finding the Molarity of the Reactant
Homework Statement 96 grams of Iron(III)Chloride is dissolved in water to give 300ml of solution. What is the Molarity of Iron(III)Chloride? What is the Molarity of just the Chloride? Homework Equations FeCl3 + H2O → Fe+3 + 3Cl- The Attempt at a Solution 96g FeCl3 X (1 mol FeCl3 /...- derwalrus
- Thread
- Molarity
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
D
Help Solving Percent Molarity Problem
I have to prepare the following solution: 200 mL of 2.5% Glutaraldehyde from 25% stock Glutaraldehyde in 0.2M Sodium Cacodylate Buffer So far I know: Molecular weight of Glutaraldehyde: 100g/mol and Sodium Cacodylate:160g/mol Assuming the density is 1 of Glutaldehyde, then...- depeche
- Thread
- Molarity Percent
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
B
Finding volume using Molarity + Equation
Homework Statement In the photographic process silver bromide is dissolved by adding sodium thiosulfate. Determine the volume in mL of 0.0138 M Na2S2O3 should be added to dissolve 0.250 g of AgBr. Homework Equations The equation is : AgBr (s) + Na2S2O3 (aq) → Na3Ag(S2O3)2 (aq) + NaBr...- ballinbob
- Thread
- Molarity Volume
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
P
Titration of thiosulphate with KIO3, molarity? PLEASE HELP
Homework Statement I am so confused. I am doing a "Iodometric Titration of Copper in Brass" lab. We first made a thiosulphate solution by mixing 500 mL water, 12.5 g of Na2S203 * 5H20, and .05 g Na2CO3. This solution is "supposed" to come out to be about 0.1 M. My actual measurements...- Puchinita5
- Thread
- Molarity Titration
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
S
Make 1L Culture Media: 10X Liquid Salt & FBS
Homework Statement You need to prepare medium for your culture cells. Your liquid salt solution is 10X concentration, you need to dilute to 1X for use. You need to add 100% fetal bovine serum for a final concentration of 10%. How would you make up 1 liter of this culture media using water as...- Simon777
- Thread
- Calculation Molarity
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
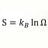
Maximum concentration, molarity, of Carbonic Acid ?
What is the maximum concentration, Moles/liter, possible for one liter of H2CO3 at STP. And how is that calculated ? -
W
Chem: Calculate volume given molarity and pH
Homework Statement What volume of solution is needed to dissolve 1.0 mol of a strong base such as KOH to make a solution whose pH is 12.5? Homework Equations KOH_{(s)} \rightarrow K^{+}_{(aq)} + OH^{-}_{(aq)} pH = -log[H_{3}O^{+}] The Attempt at a Solution I'm not going to...- Winning
- Thread
- Chem Molarity Ph Volume
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
G
Comparing Molality & Molarity: Which is More Concentrated?
hello, i had been comparing the molarity and molality of a solution. i know molarity is moles of solute per L of solution, and molality is moles of solute per kg of solvents. but what i m interested to know is- which of the two is more concentrated- 1molal solution or 1 molar solution. my...- Garvit Goel
- Thread
- Molality Molarity
- Replies: 2
- Forum: Chemistry