- #1
1832vin
- 58
- 1
This is the question and the answer, the problem is i don't know how to get to the answer
top is question and bottom is answer
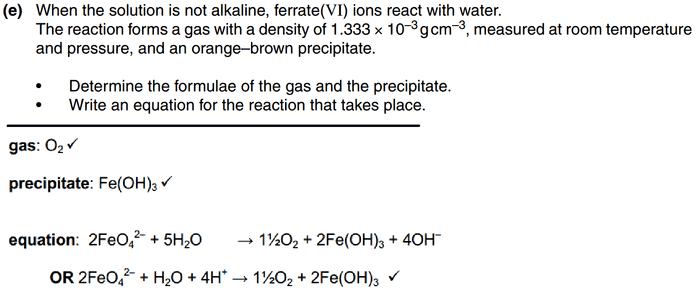
I attempted to divide the gas concentration by 24 to get molar mass, but that didn't work
and how am i suppose to know that OH- is one of the products? i guessed that there is fe(OH)3, but that's all i got right...
it's the very last question from this A level paper
please help, my exams are close, and i have no clue at all...
top is question and bottom is answer
I attempted to divide the gas concentration by 24 to get molar mass, but that didn't work
and how am i suppose to know that OH- is one of the products? i guessed that there is fe(OH)3, but that's all i got right...
it's the very last question from this A level paper
please help, my exams are close, and i have no clue at all...