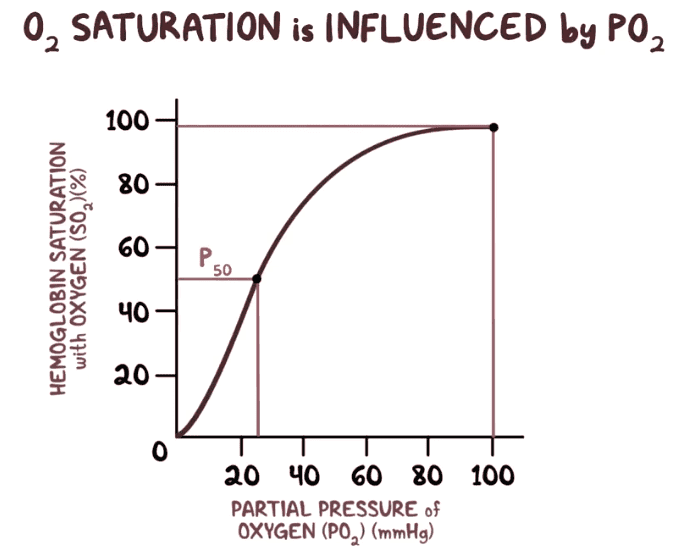
It gets easier to bind when some oxygen is already bound but on the other hand there are fewer empty sites to bind to, making binding more difficult if we speak loosely.
So let's not speak loosely, let's speak physically, thermodynamically – the only physically meaningful measure of "ease of binding" is an equilibrium constant:
K = [unoccupied sites][oxygen concentration]/[unoccupied sites] often written
$$ K = \frac {X}{x(1 - X)}$$
where ##X## is the saturation (= fraction of sites occupied by oxygen or other ligand, ##x## is the ligand solution concentration.) The above expression is an equilibrium constant, K is an association constant - which, er, varies as a function of saturation if you have cooperativity.
If I were teaching the subject I would not mention the word sigmoid at all, except as warning. It leads to confusion and even wrong statements sometimes, but the term is very frequently used in introductions to the subject.
Actually binding data is not so often reported as saturation saturation against ligand solution concentration (or equivalent lead here as gas pressure which ideally is proportional to solution concentration*); rather it is reported in the "Hill Plot" as
##log \frac{ X}{1-X}## against ##log x##
If there is no cooperativity the slope of the plot is exactly 1, where there is positive cooperativity it is higher.
You might find helpful to to consider a family of non-cooperative learning curves binding curves. Obeying, that is, the equation
$$ X = \frac {x}{K + x}$$
The lower K, the lower the ligand concentration needed to fill the binding sites. If you look at an individual curve you might naïvely say binding gets more difficult in each of them the more that is already bound. But from our definition of difficulty, they have the same equilibrium binding constant at all points along a given curve. On the other hand a cooperative curve (red) if it has a point in common with one noncooperative curve (blue), its next - higher concentration - points will intersect other curves corresponding to higher affinity.