Plane Wave
- 8
- 0
Why are absorption cross sections for atoms so much larger than that of molecules.
For example, the absorption cross section for the D2 line in Rubidium is ~1E-9 cm^2. Specifically, the cross section is basically σ~λ^2
The absorption cross sections for say, I2, is 9 orders of magnitude smaller! Briefly looking through many of the molecular spectra, all of the cross sections are on order of 10E-18 cm^2 to 10E-19 cm^2.
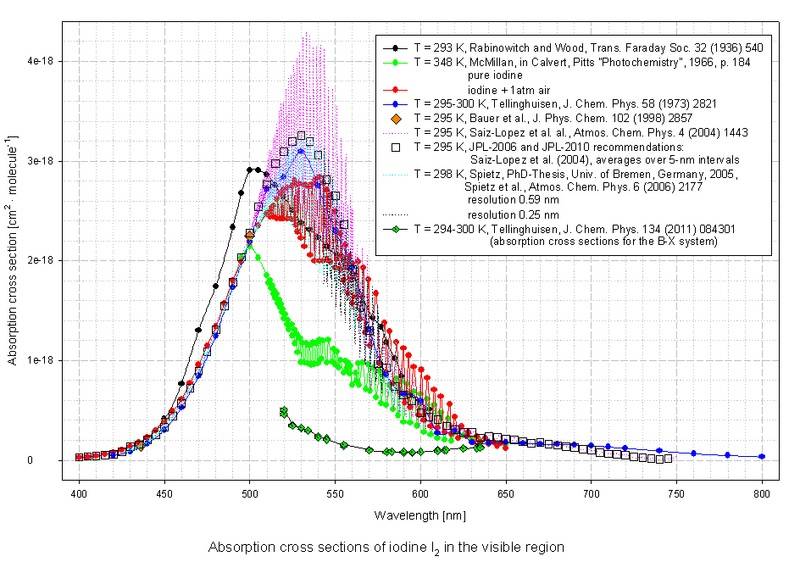
http://joseba.mpch-mainz.mpg.de/spe.../Halogens+mixed halogens/I2_400-800nm_lin.jpg
Does anyone know why the absorption cross section for molecular transitions are so much smaller than the atomic absorption cross section?
For example, the absorption cross section for the D2 line in Rubidium is ~1E-9 cm^2. Specifically, the cross section is basically σ~λ^2
The absorption cross sections for say, I2, is 9 orders of magnitude smaller! Briefly looking through many of the molecular spectra, all of the cross sections are on order of 10E-18 cm^2 to 10E-19 cm^2.
http://joseba.mpch-mainz.mpg.de/spe.../Halogens+mixed halogens/I2_400-800nm_lin.jpg
Does anyone know why the absorption cross section for molecular transitions are so much smaller than the atomic absorption cross section?