- #1
Elbow_Patches
- 4
- 0
- TL;DR Summary
- What thickness of lead would you expect a strontium-90 beta source to penetrate?
Hello everyone,
We conducted an experiment with a strontium-90 source and some different thicknesses of lead.
With 2.1mm of lead the count rate (corrected for the background) was 0.69 counts per second,
3.0mm 19.7cps
6.8mm 15.4cps
13.8mm 10.0 cps
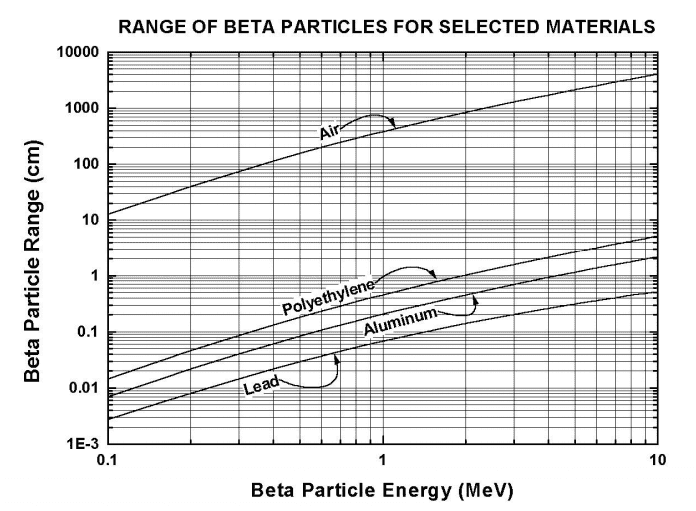
This would indicate that the thicker the lead, the more the absorption, the lower the count. Which makes sense, but shouldn't the 2.1mm have been thick enough to stop all beta particles from passing through? According to some data I've found (figure 1) for a beta particle to penetrate 1mm of lead would require an energy of about 1-2 MeV, when you'd expect an Sr-90 source to emit them with about 0.5MeV.
So, what do you think? Are these results normal and to be expected (not the opinion of others in my department)? Has the strontium decayed into something that emits gamma? Has the strontium decayed into yttrium which then emits gamma with about 2.3MeV (so according to figure 1 it would penetrate through 1-2mm of lead)? Is the beta striking the lead and producing bremsstrahlung radiation? Something else I haven't thought of?
Any help, suggestions or musings are welcome. :)
We conducted an experiment with a strontium-90 source and some different thicknesses of lead.
With 2.1mm of lead the count rate (corrected for the background) was 0.69 counts per second,
3.0mm 19.7cps
6.8mm 15.4cps
13.8mm 10.0 cps
This would indicate that the thicker the lead, the more the absorption, the lower the count. Which makes sense, but shouldn't the 2.1mm have been thick enough to stop all beta particles from passing through? According to some data I've found (figure 1) for a beta particle to penetrate 1mm of lead would require an energy of about 1-2 MeV, when you'd expect an Sr-90 source to emit them with about 0.5MeV.
So, what do you think? Are these results normal and to be expected (not the opinion of others in my department)? Has the strontium decayed into something that emits gamma? Has the strontium decayed into yttrium which then emits gamma with about 2.3MeV (so according to figure 1 it would penetrate through 1-2mm of lead)? Is the beta striking the lead and producing bremsstrahlung radiation? Something else I haven't thought of?
Any help, suggestions or musings are welcome. :)
