- #1
Mohankpvk
- 102
- 3
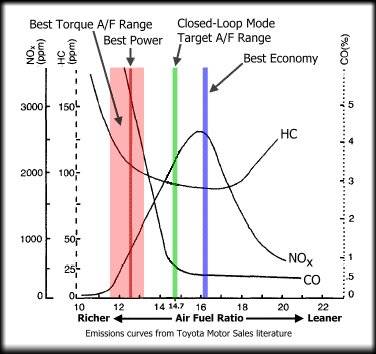
How can the slightly richer Air fuel ratio produce higher power while the temperature it produces is lesser than slightly leaner(slightly leaner than stoichiometric for best economy)?
FYI:I understood that ,at the stoichiometric ratio(precisely slightly leaner due to fast operation of engine) there will be enough oxygen for all the fuel to be burnt completely.So it results in higher temperature.
In case of a richer mixture, lesser than required oxygen combined with the cooling effect of the fuel(enthalpy of vapourisation), the temperature is lower.
But my doubt is,how can a lower temperature produce the best power(maximum)?
I thought, higher temperature=higher pressure=higher power.
I have attached a graph (I found on the internet).
FYI:I understood that ,at the stoichiometric ratio(precisely slightly leaner due to fast operation of engine) there will be enough oxygen for all the fuel to be burnt completely.So it results in higher temperature.
In case of a richer mixture, lesser than required oxygen combined with the cooling effect of the fuel(enthalpy of vapourisation), the temperature is lower.
But my doubt is,how can a lower temperature produce the best power(maximum)?
I thought, higher temperature=higher pressure=higher power.
I have attached a graph (I found on the internet).
Attachments
Last edited:

