GravityX
- 19
- 1
- Homework Statement
- Apply Legendre Transformation to Entropy
- Relevant Equations
- ##g(m)=f(x(m))-m*x(m)## and ##x(m)=(f')^-1(m)##
Hi,
Unfortunately I am not getting anywhere with task three, I don't know exactly what to show
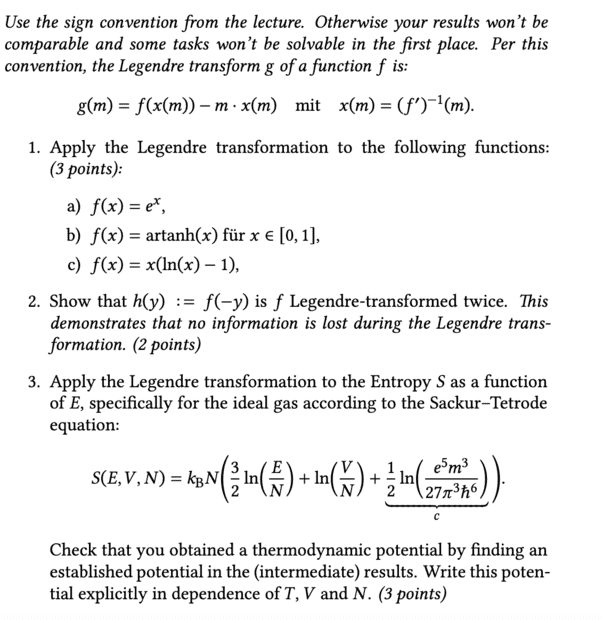
Shall I now show that from ##S(T,V,N)## using Legendre I then get ##S(E,V,N)## and thus obtain the Sackur-Tetrode equation?
Unfortunately I am not getting anywhere with task three, I don't know exactly what to show
Shall I now show that from ##S(T,V,N)## using Legendre I then get ##S(E,V,N)## and thus obtain the Sackur-Tetrode equation?