- #1
silenzer
- 54
- 0
I have two problems.
How many moles of Na2CO3 must be dissolved in 250 mL of 0,125 M NaHCO3 so that the solution has a pH of 10,00?

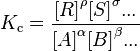
The powers of the concentrations are the coefficients in the chemical equation. The products of the equation are in the numerator, and the reactants in the denominator.
I have an idea for a solution, but I have no way of checking whether it's correct. My solution is as follows.
The sodium ions can be ignored because they have no significant effect on the pH.
The equation is: HCO3- ⇔ H+ + CO32-
I set [CO3]2-=y, and to equilibrium this concentration changes to y+x, but I assume that x is very small compared to y so y+x ≈ y. The same applies to HCO32-. The initial concentration of that is 0,5 M, and it occurs change towards equilibrium that is 0,5 M - x, which is essentially 0,5 M.
If I'm allowed to do the above, the problem is easy. I know Ka for HCO32- so all I have to do is plug everything I know into the Ka equation and solve for [H+].
In a very basic solution the insoluble salt Cr(OH)3 (Ksp=1,6*10-30) dissolves and forms the metal complex Cr(OH)4- (Kf=8*1028). Calculate the equilibrium constant, K, for the reaction:
Cr(OH)3(s) + OH-(aq) ⇔ Cr(OH)4-(aq).
Same as in the first problem.
I'm not sure how to approach this exact problem. I know how to solve simpler problems that are showcased in my textbook, but there you're given numbers like molarities to work with. Here I only have equilibrium constants.
Homework Statement
How many moles of Na2CO3 must be dissolved in 250 mL of 0,125 M NaHCO3 so that the solution has a pH of 10,00?
Homework Equations
The powers of the concentrations are the coefficients in the chemical equation. The products of the equation are in the numerator, and the reactants in the denominator.
The Attempt at a Solution
I have an idea for a solution, but I have no way of checking whether it's correct. My solution is as follows.
The sodium ions can be ignored because they have no significant effect on the pH.
The equation is: HCO3- ⇔ H+ + CO32-
I set [CO3]2-=y, and to equilibrium this concentration changes to y+x, but I assume that x is very small compared to y so y+x ≈ y. The same applies to HCO32-. The initial concentration of that is 0,5 M, and it occurs change towards equilibrium that is 0,5 M - x, which is essentially 0,5 M.
If I'm allowed to do the above, the problem is easy. I know Ka for HCO32- so all I have to do is plug everything I know into the Ka equation and solve for [H+].
Homework Statement
In a very basic solution the insoluble salt Cr(OH)3 (Ksp=1,6*10-30) dissolves and forms the metal complex Cr(OH)4- (Kf=8*1028). Calculate the equilibrium constant, K, for the reaction:
Cr(OH)3(s) + OH-(aq) ⇔ Cr(OH)4-(aq).
Homework Equations
Same as in the first problem.
The Attempt at a Solution
I'm not sure how to approach this exact problem. I know how to solve simpler problems that are showcased in my textbook, but there you're given numbers like molarities to work with. Here I only have equilibrium constants.