EE18
- 112
- 13
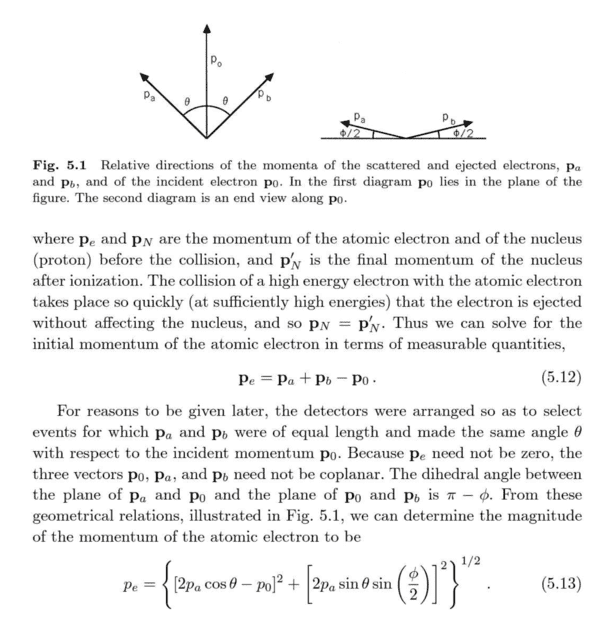
In Chapter 5.3, Ballentine uses geometrical arguments to obtain the initial magnitude of a hydrogen atom's bound electron momentum. How does equation (5.13) obtain? I tried to naively compute
$$p_e^2 \equiv \textbf{p}_e\cdot \textbf{p}_e = p_a^2+p_b^2+p_o^2 + 2\textbf{p}_a\cdot \textbf{p}_b - 2\textbf{p}_a\cdot \textbf{p}_0 - 2\textbf{p}_0\cdot \textbf{p}_b $$ $$= p_a^2+p_b^2+p_o^2 + 2p_ap_b\cos(\pi - \phi) - 2p_ap_0\cos \theta - 2p_bp_0\cos \theta$$
but then could not go any further. Am I misunderstanding the geometrical relationships of the vectors in Figure 5.1?
$$p_e^2 \equiv \textbf{p}_e\cdot \textbf{p}_e = p_a^2+p_b^2+p_o^2 + 2\textbf{p}_a\cdot \textbf{p}_b - 2\textbf{p}_a\cdot \textbf{p}_0 - 2\textbf{p}_0\cdot \textbf{p}_b $$ $$= p_a^2+p_b^2+p_o^2 + 2p_ap_b\cos(\pi - \phi) - 2p_ap_0\cos \theta - 2p_bp_0\cos \theta$$
but then could not go any further. Am I misunderstanding the geometrical relationships of the vectors in Figure 5.1?