- #1
mukul
- 17
- 2
There had been a big time debate in my college between chemistry faculties and physics faculties on whether the following process is reversible or not.
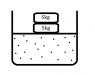
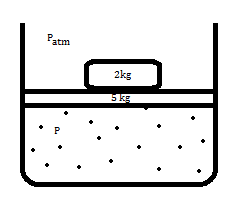
consider that there is some gas (assuming ideal gas) in a chamber with friction less piston of 5kg and 2 kg mass on top of it as shown in the figure. let's say the system is in equilibrium ie pressure of the gas balances 7 kg weight.
We now remove 2kg block and due to which system gas will expand.
Will the expansion of gas reversible or irreversible.
According to physics faculties, this is reversible (assuming ideal gas and friction less piston) since the situation will be just like a spring block system. when the gas will expand then the work done on 5kg piston will be (p(t)-p(atm))*A - mg (here m is mass of piston ie 5kg) and this will be the kinetic energy gained by the 5kg piston. Due to this kinetic energy the piston will further expand to reach at the level where its kinetic energy is zero. The piston will now come down upto the same level from where it started.
Any opinion is highly appreciated.
consider that there is some gas (assuming ideal gas) in a chamber with friction less piston of 5kg and 2 kg mass on top of it as shown in the figure. let's say the system is in equilibrium ie pressure of the gas balances 7 kg weight.
We now remove 2kg block and due to which system gas will expand.
Will the expansion of gas reversible or irreversible.
According to physics faculties, this is reversible (assuming ideal gas and friction less piston) since the situation will be just like a spring block system. when the gas will expand then the work done on 5kg piston will be (p(t)-p(atm))*A - mg (here m is mass of piston ie 5kg) and this will be the kinetic energy gained by the 5kg piston. Due to this kinetic energy the piston will further expand to reach at the level where its kinetic energy is zero. The piston will now come down upto the same level from where it started.
Any opinion is highly appreciated.