- #1
desmond iking
- 284
- 2
Homework Statement
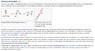
for the bromination of alkene, different sources give different structure of product which is trans(from wikipedia) , but the book is cis. which is correct?