mathlearn
- 331
- 0
View attachment 6158
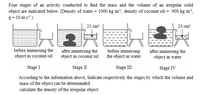
Stage II and stage IV can be used to determine as the volume of the water displaced is given.
Now how should the density be calculated :D
I know the formula for density
$density=\frac{mass}{volume}$
Stage II and stage IV can be used to determine as the volume of the water displaced is given.
Now how should the density be calculated :D
I know the formula for density
$density=\frac{mass}{volume}$