- #1
Suy
- 101
- 0
Homework Statement
I just have a quiz, but i don't get this question
this is all i can remember
A=-4.0*10^-6C
B=+2.5*10^-6C
mass of sphere: 51g
initial separated distance:10.0cm
If A and B touch and then repel, what is the new separated distance
After they touch,
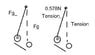
Tension force on the string is 0.578N
this is the picture after they touch
http://img10.imageshack.us/img10/5527/26792246.jpg
Homework Equations
Fe=(kq1q2)/r2
F=mg?
The Attempt at a Solution
I don't get this question at all, but i know i need to find the force first...
After they touch, the electron divide equally, so +2.5*10^-6 neutralize -2.5*10^-6, 1.5*10^-6 left, and divide by 2 is 0.75*10^-6
am i right?
Last edited by a moderator: