Dario56
- 289
- 48
I have a discussion with a colleague of mine.
We have a thin cuboid sample whose two dimensions are similar to each other and are both much bigger than the sample thickness. I'm doing an experiment in which the diffusion of some species is induced and its diffusion profile is measured in one of the sample directions. Diffusion profile is fitted to the solutions of the diffusion equation (Fick's 2nd law) to obtain material properties.
Since the two dimensions of the sample are similar and I'm only measuring the diffusion profile in one of these two directions, is it meaningful to fit this profile to the one-dimensional diffusion equation solution? If you ask me, the answer is no. My colleague claims this can be done.
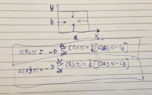
The problem is that the diffusion rate (molar flux) is similar in both dimensions (directions) and therefore concentration time change at any point is affected by the fluxes in both directions. Hence, we must measure the concentration profile over the sample surface and fit it to the two-dimensional solution.
Who do you think is right here? Can diffusion be modelled as one-dimensional?
We have a thin cuboid sample whose two dimensions are similar to each other and are both much bigger than the sample thickness. I'm doing an experiment in which the diffusion of some species is induced and its diffusion profile is measured in one of the sample directions. Diffusion profile is fitted to the solutions of the diffusion equation (Fick's 2nd law) to obtain material properties.
Since the two dimensions of the sample are similar and I'm only measuring the diffusion profile in one of these two directions, is it meaningful to fit this profile to the one-dimensional diffusion equation solution? If you ask me, the answer is no. My colleague claims this can be done.
The problem is that the diffusion rate (molar flux) is similar in both dimensions (directions) and therefore concentration time change at any point is affected by the fluxes in both directions. Hence, we must measure the concentration profile over the sample surface and fit it to the two-dimensional solution.
Who do you think is right here? Can diffusion be modelled as one-dimensional?