vcsharp2003
- 913
- 179
Homework Statement:: I am trying to understand a formula given in our book for determining molar heat capacity of an ideal gas under different thermodynamic processes using a single formula, but it is confusing. The exact formula for different processes is in the screenshots below. Can someone please explain how ##x## is calculated for different processes? The power of x is confusing me.
Relevant Equations:: As given in screenshot
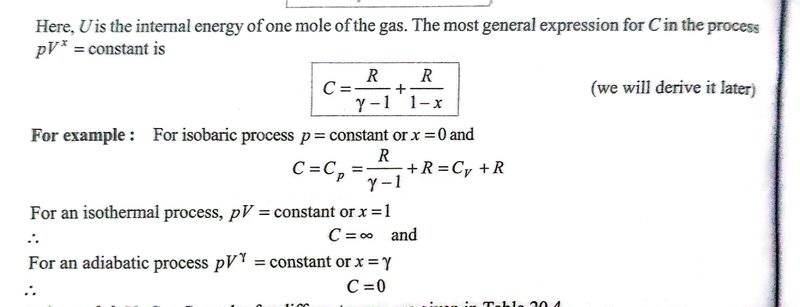
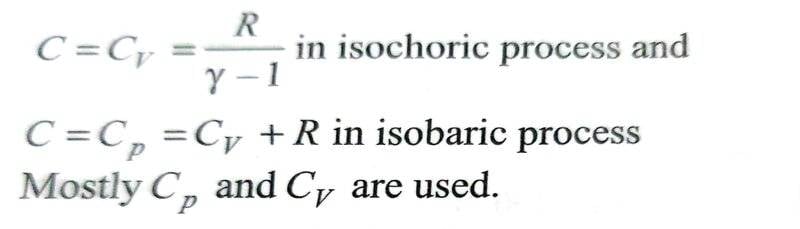
To apply the formula for molar heat capacity using the equation mentioned above, one needs to first determine ##x## value and plug it into the molar heat capacity formula. But I am hitting a roadblock in finding ##x## for certain processes like isochoric, isothermal and adiabatic processes. I do get it for isobaric process.
The equation ##PV^x = constant ## doesn't make sense to me for isochoric process, in which ##V## is constant. To satisfy the above equation. ##x## would need to take a value so the left side of the equation is constant i.e. as ##P## increases, ##V^x## should decrease making ##x## take a variable set of multiple values. If, we can conclude that ##x= \infty ## then the second term on right hand side will be ##\frac {R}{1- \infty} = 0##, then the formula makes sense for isochoric process.
For isobaric process I get it. Since ##P## is already constant, so we need to only make sure that ##V^x## is constant, which is only true if ##x=0##.
Relevant Equations:: As given in screenshot
To apply the formula for molar heat capacity using the equation mentioned above, one needs to first determine ##x## value and plug it into the molar heat capacity formula. But I am hitting a roadblock in finding ##x## for certain processes like isochoric, isothermal and adiabatic processes. I do get it for isobaric process.
The equation ##PV^x = constant ## doesn't make sense to me for isochoric process, in which ##V## is constant. To satisfy the above equation. ##x## would need to take a value so the left side of the equation is constant i.e. as ##P## increases, ##V^x## should decrease making ##x## take a variable set of multiple values. If, we can conclude that ##x= \infty ## then the second term on right hand side will be ##\frac {R}{1- \infty} = 0##, then the formula makes sense for isochoric process.
For isobaric process I get it. Since ##P## is already constant, so we need to only make sure that ##V^x## is constant, which is only true if ##x=0##.
Last edited: