- #1
SCalver
- 1
- 0
Hi guys,
I'm doing a project at the moment revolving around capillary action and surface tension. Today I conducted an experiment to observe capillary action in a capillary tube and paper towel at different temperatures of water. I don't understand why I've got the following results:
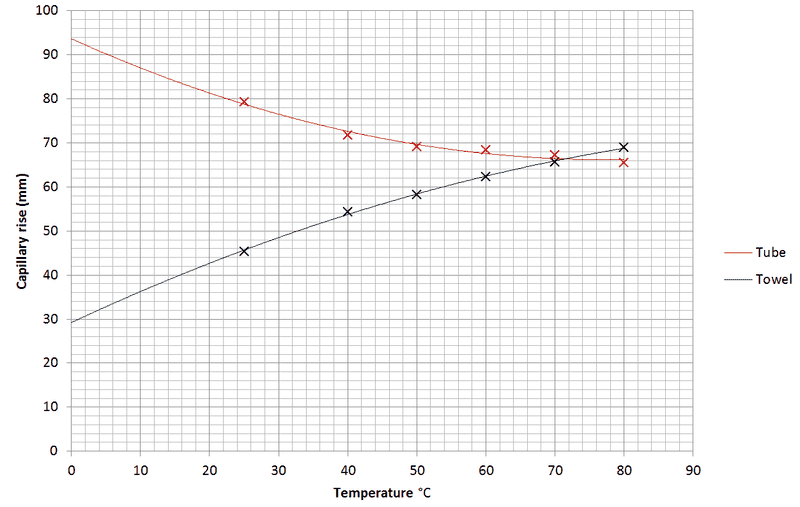
As you can see, they are complete opposites of each other. This seems to make sense according to my calculations with Washburns and Jurins equations. But I can't figure out the physics behind this. The capillary tube radius is 0.2mm. The paper towel was 1.5x23cm with 5cm submerged in water for a time of 120 seconds.
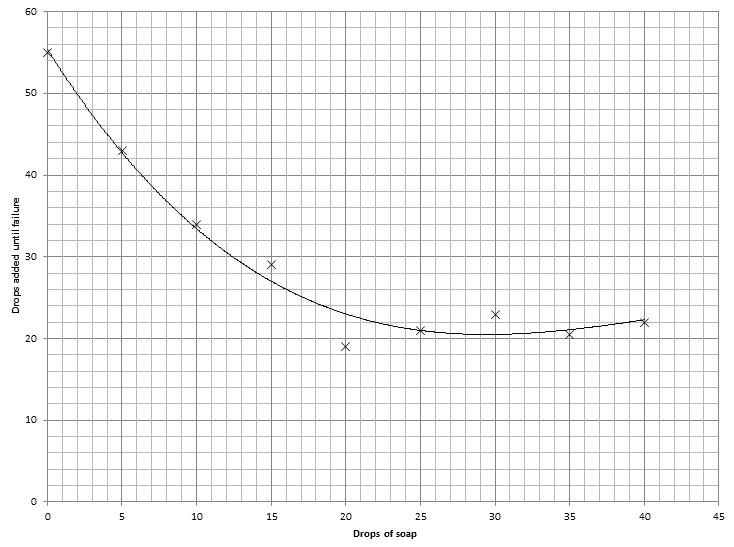
I also did an experiment investigating the relation between surface tension and soapy water. I tried different concentrations of soap solution and added them dropwise onto the top of a circular metal disk very carefully until failure occured. Any ideas why my graph seems to level out after while?:
here is a picture of what I mean by failure (excuse my crappy camera)
non failure:

Failure:

any help appreciated
I'm doing a project at the moment revolving around capillary action and surface tension. Today I conducted an experiment to observe capillary action in a capillary tube and paper towel at different temperatures of water. I don't understand why I've got the following results:
As you can see, they are complete opposites of each other. This seems to make sense according to my calculations with Washburns and Jurins equations. But I can't figure out the physics behind this. The capillary tube radius is 0.2mm. The paper towel was 1.5x23cm with 5cm submerged in water for a time of 120 seconds.
I also did an experiment investigating the relation between surface tension and soapy water. I tried different concentrations of soap solution and added them dropwise onto the top of a circular metal disk very carefully until failure occured. Any ideas why my graph seems to level out after while?:
here is a picture of what I mean by failure (excuse my crappy camera)
non failure:
Failure:
any help appreciated