- #1
Devin Longo
- 11
- 0
Homework Statement
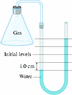
An experiment to determine the specific heat of a gas makes use of a water manometer attached to a flask (the figure below ). Initially the two columns of water are even. Atmospheric pressure is 1.5 105 Pa. After heating the gas, the water levels change to those shown. Find the change in pressure of the gas in Pa.
2. Homework Equations
[tex]\Delta[/tex]P = [tex]\rho[/tex]g[tex]\Delta[/tex]h
The Attempt at a Solution
I basically did 1.5 x 10 ^5 * 9.8 m/s2 * .01m (what I assume given the picture to be the change in height)