lorenz0
- 151
- 28
- Homework Statement
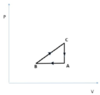
- A thermodynamic cycle has ##n=40 mol## of ideal diatomic gas, initially at (A) with ##P_A=1.0*10^5 Pa## and ##V_A=4m^3##, then goes to (B) via an isobaric compression such that ##V_B=\frac{V_A}{2}##, followed by an expansion described by ##P=k\cdot V## such that ##V_C=V_A=4m^3## and followed by an isocorci transformation that brings the gas back to state (A).
(a) Find the work done by the gas in one cycle, the heat from the gas to the environment in a cycle and the efficiency of the engine;
(b) Suppose now the engine can exchange heat with two sources: it gives off heat to the environment at temperature ##T_{env}=300K## and absorbs heat from a source at ##T_{s}=6000K.## Find out if the machine is reversible or irreversible and determine the variation of the entropy of the universe in one cycle.
- Relevant Equations
- ##W=-p\Delta V##, ##Q=nC_V \Delta T##, ##\eta=1-\frac{T_c}{T_h}##, ##PV=nRT##
(a) We first find that: ##T_A=\frac{P_A V_A}{nR}=\frac{1\cdot 10^5 \cdot 4}{40\cdot 8.314}K\approx 1202.7904 K##, ##\frac{T_B}{T_A}=\frac{\frac{P_B V_B}{nR}}{\frac{P_A V_A}{nR}}=\frac{P_B V_B}{P_A V_A}=\frac{P_A \frac{V_A}{2}}{P_A V_A}=\frac{1}{2}##, ##\frac{T_C}{T_B}=\frac{P_C V_C}{nR}\frac{nR}{P_B V_B}=\frac{kV_C V_C}{kV_B V_B}=\frac{kV_A V_A}{k \frac{V_A}{2}\frac{V_A}{2}}=4## so ##T_C=4T_B=4\frac{T_A}{2}=2T_A##. Also ##P_B=\frac{nRT_B}{V_B}=\frac{nR\frac{T_A}{2}}{\frac{V_A}{2}}=\frac{nRT_A}{V_A}=P_A=\frac{40\cdot 8.314\cdot 1202.7904}{4}Pa\approx 100,000 Pa##, ##P_C=\frac{nRT_C}{V_C}=\frac{nR\cdot 2T_A}{V_A}=\frac{40\cdot 8.314\cdot 2\cdot 1202.7904}{4}Pa\approx 200,000 Pa## so ##k=\frac{P_C-P_B}{V_C-V_B}=\frac{200,000-100,000}{4-2}\frac{Pa}{m^3}=50,000 \frac{Pa}{m^3}.## We also note that, the gas being diatomic, it is ##C_P=\frac{7}{2}R## and ##C_V=\frac{5}{2}R.##
#A\to B:## ##W_{A\to B}=-P_A(V_B-V_A)=200,000\ Joule##, Q_{A\to B}=nC_P(T_B-T_A)=-700,000\ Joule.##
##B\to C:## ##W_{B\to C}=\int_{V_B}^{V_C}P dV=\int_{V_B}^{V_C}kV dV=\frac{k}{2}(V_C^2-V_B^2)=300,000\ Joule.##
## Q_{B\to C}=\Delta U-W_{B\to C}=nC_V(T_C-T_B)-W_{B\to C}=1,200,000\ Joule.##
##C\to A:## ##W_{C\to A}=0\ Joule##, ##Q_{C\to A}=nC_V(T_A-T_C)=-1,000,000\ Joule.##
So the total work done is ##W_{TOT}=(200,000+300,000)\ Joule=500,000\ Joule##, the heat given to the environment ##Q_{g}=(700,000+1,000,000)\ Joule=1,700,000\ Joule## and the efficiency is ##\eta=\frac{W_{done}}{Q_{absorbed}}=\frac{200,000+300,000}{1,200,000}=\frac{5}{24}.##
(b) With the given sources temperatures we have that ##\eta_{ideal}=1-\frac{T_{cold}}{T_{hot}}=1-\frac{300}{6000}=\frac{19}{20}>\eta## so the engine is not ideal (irreversible).My question now is: how do I find the change in entropy of the universe? I know how to find the entropy change in each step of the cycle by applying ##\Delta S=nC_V\ln(\frac{T_f}{T_i})+nR\ln(\frac{V_f}{V_i})## but how do I go from that to finding the entropy change of the Universe? Thanks.
#A\to B:## ##W_{A\to B}=-P_A(V_B-V_A)=200,000\ Joule##, Q_{A\to B}=nC_P(T_B-T_A)=-700,000\ Joule.##
##B\to C:## ##W_{B\to C}=\int_{V_B}^{V_C}P dV=\int_{V_B}^{V_C}kV dV=\frac{k}{2}(V_C^2-V_B^2)=300,000\ Joule.##
## Q_{B\to C}=\Delta U-W_{B\to C}=nC_V(T_C-T_B)-W_{B\to C}=1,200,000\ Joule.##
##C\to A:## ##W_{C\to A}=0\ Joule##, ##Q_{C\to A}=nC_V(T_A-T_C)=-1,000,000\ Joule.##
So the total work done is ##W_{TOT}=(200,000+300,000)\ Joule=500,000\ Joule##, the heat given to the environment ##Q_{g}=(700,000+1,000,000)\ Joule=1,700,000\ Joule## and the efficiency is ##\eta=\frac{W_{done}}{Q_{absorbed}}=\frac{200,000+300,000}{1,200,000}=\frac{5}{24}.##
(b) With the given sources temperatures we have that ##\eta_{ideal}=1-\frac{T_{cold}}{T_{hot}}=1-\frac{300}{6000}=\frac{19}{20}>\eta## so the engine is not ideal (irreversible).My question now is: how do I find the change in entropy of the universe? I know how to find the entropy change in each step of the cycle by applying ##\Delta S=nC_V\ln(\frac{T_f}{T_i})+nR\ln(\frac{V_f}{V_i})## but how do I go from that to finding the entropy change of the Universe? Thanks.