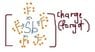- #1
coconut62
- 161
- 1
There's a question in my class test paper today which requires us to draw the dot-and-cross diagram for SbF5.
There's a charge on that molecule.
I drew it like this: (attached image)
Where does the charge come from? I don't even see any extra electrons. :shy:
There's a charge on that molecule.
I drew it like this: (attached image)
Where does the charge come from? I don't even see any extra electrons. :shy: