- #1
mishima
- 576
- 43
Hi, I have an older spectrophotometer (Spectronic 20) I've been trying to get up and running mostly just for fun, but also to learn more about spectrophotometry in general.
Recently I tried constructing an absorbance spectrum for chlorophyll in acetone. I took an unmeasured amount of cuttings from a plant in my room and put them in (hardware store bought) acetone. I used roughly 100mL.
I then made 2 cuvettes, 1 just acetone, and one the filtered liquid from the cuttings. I tried going through the range of wavelengths, recalibrating 0 absorbance each change of wavelength with the acetone cuvette and reading absorbance every 10 nm with the cuttings cuvette.
Question: some wavelengths I could not calibrate because the absorbance would never reach zero, even with the dial turned all the way (why?). I thus only have readings for 380-600 nm. This is disappointing because I think the wavelength of interest is 650+. From Beer's law it looks like a solution would be to dilute the acetone in both cuvettes, or use a smaller cuvette (which I don't have).
I plotted absorbance vs. wavelength in excel. It's a nice curve but it doesn't seem to mesh with other absorbance spectra I found kicking around on the net.
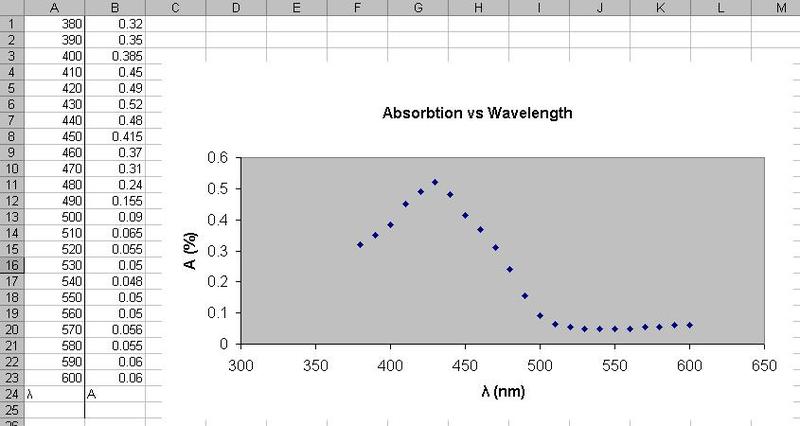
Question: any interpretations of my graph? Is the peak near 430 why the solution fluoresces in black light?
I really appreciate any help.
edit: and I just realized I misspelled "absorption" on my graph...
Recently I tried constructing an absorbance spectrum for chlorophyll in acetone. I took an unmeasured amount of cuttings from a plant in my room and put them in (hardware store bought) acetone. I used roughly 100mL.
I then made 2 cuvettes, 1 just acetone, and one the filtered liquid from the cuttings. I tried going through the range of wavelengths, recalibrating 0 absorbance each change of wavelength with the acetone cuvette and reading absorbance every 10 nm with the cuttings cuvette.
Question: some wavelengths I could not calibrate because the absorbance would never reach zero, even with the dial turned all the way (why?). I thus only have readings for 380-600 nm. This is disappointing because I think the wavelength of interest is 650+. From Beer's law it looks like a solution would be to dilute the acetone in both cuvettes, or use a smaller cuvette (which I don't have).
I plotted absorbance vs. wavelength in excel. It's a nice curve but it doesn't seem to mesh with other absorbance spectra I found kicking around on the net.
Question: any interpretations of my graph? Is the peak near 430 why the solution fluoresces in black light?
I really appreciate any help.
edit: and I just realized I misspelled "absorption" on my graph...