- #1
Ninnec
- 1
- 0
- TL;DR Summary
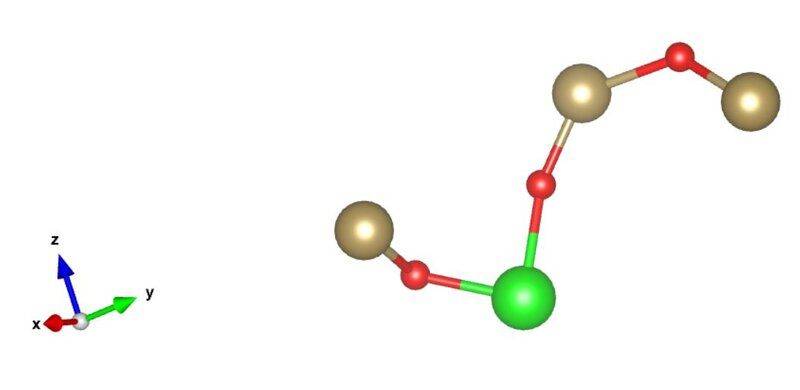
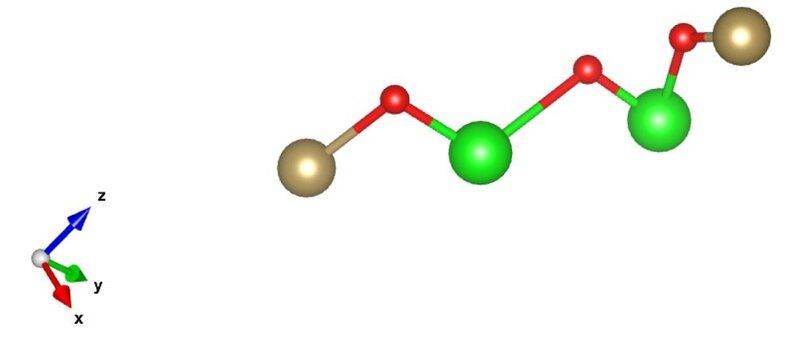
- I am trying to characterize chains of different length found in amorphous structures. I right now trying to characterize them in two different ways. The first one is how kinky or complex the chain is. The other way is to identify if this is a three dimensional, two dimensional structure. For example a chain that is a straight is a one dimensional structure. I do realize these are vague metrics/questions, I am just trying to come up with a few ideas to test. Thanks for all the help
Here are two examples of chains. The end pairs of atoms are 8.0 angstroms and 9.2 angstroms apart respectively.