- #1
Johnson
- 29
- 0
Structures of Glycine at pH's 1, 5.97 and 13, just wondering if anyone can confirm these, been a while since molecular bio.
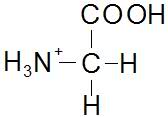
pH 1

pH 6
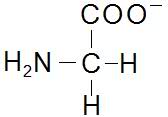
pH 13
Regards,
Andrew Johnson
pH 1
pH 6
pH 13
Regards,
Andrew Johnson
Last edited: