EddiePhys
- 144
- 6
So I'm reading through Cengel's thermodynamics textbook, and came across this solved example:
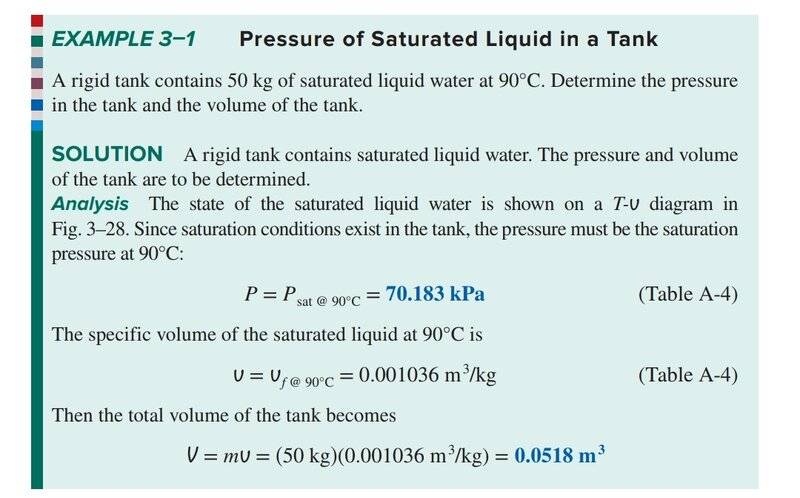
Firstly, pressure in this context I'm assuming is vapour pressure? Since we're dealing with pure substances in this chapter.
But what's confusing me is, here's the diagram I have:
They've not mentioned that the water fills the tank. So I don't understand why the specific volume times the mass of the water equals the volume of the tank?
They've used similar reasoning in following solved examples as well where it's unclear that the liquid will occupy the tank volume
Firstly, pressure in this context I'm assuming is vapour pressure? Since we're dealing with pure substances in this chapter.
But what's confusing me is, here's the diagram I have:
They've not mentioned that the water fills the tank. So I don't understand why the specific volume times the mass of the water equals the volume of the tank?
They've used similar reasoning in following solved examples as well where it's unclear that the liquid will occupy the tank volume
Last edited: