member 731016
- Homework Statement
- Please see below
- Relevant Equations
- Please see below
For this,
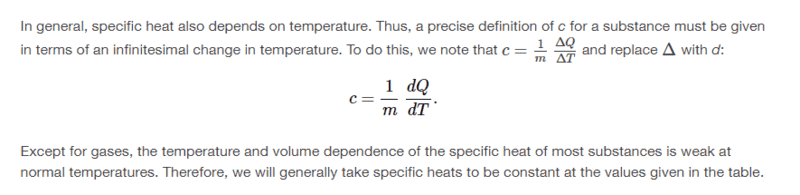
Dose anybody please know of a better way to derive the formula without having ##c = \frac{\Delta Q}{m \Delta T}## then taking the limit of both sides at ##\Delta T## approaches zero? I thought ##\Delta Q## like ##\Delta W## was not physically meaningful since by definition ##Q## is the heat transfer.
Many thanks!
Dose anybody please know of a better way to derive the formula without having ##c = \frac{\Delta Q}{m \Delta T}## then taking the limit of both sides at ##\Delta T## approaches zero? I thought ##\Delta Q## like ##\Delta W## was not physically meaningful since by definition ##Q## is the heat transfer.
Many thanks!