- #1
Rukas Kang
- 1
- 0
Homework Statement
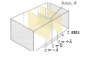
Prove that the diffusion constant D of an ideal gas can be expressed as D=1/3λv(v bar) where λ is the mean free pass of the gas molecule. And v bar is the average speed of the gas molecules obtained from the kinetic theory of gases.(Use the picture to setup the net flux change of gas molecules flowing from a negative to positive z direction.)
Homework Equations
Use the Taylor expansion of the form
f (x) = a0f(0)+ a1(df/dx)(x=0)x +...+ an(d^nf/dx^n)(x=0)x^n
for calculating the concentration c(z) on z axis at z = ±λ with the initial condition c(z=0)=c0 . When doing so, use the first two terms only of the expansion and use a1 = 2/3 for this particular system. You may need to use the molecular flux Φ (i.e. # of molecules crossing a unit area per a unit time) of the gas.
The Attempt at a Solution
I've tried to solve as deriving the the coefficient of viscosity, but I couldn't. ;(