- #1
CrimpJiggler
- 149
- 1
So when you have a cyclic diene like cyclopentadiene, you can get either the endo or exo isomer. Secondary orbital interactions make the reaction selective towards the endo isomer. This makes sense if your dieneophile is say maleic anhydride:
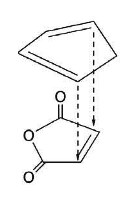
in that case the dienophile has C=O bonds which has p orbitals that will overlap with the second pair of p orbiitals on the diene.
But what if your dienophile is something like cyclopentene. In that case, the dienophile has no p oribtals for the dienophile to interact with. In this case, will the exo isomer be the major product?
in that case the dienophile has C=O bonds which has p orbitals that will overlap with the second pair of p orbiitals on the diene.
But what if your dienophile is something like cyclopentene. In that case, the dienophile has no p oribtals for the dienophile to interact with. In this case, will the exo isomer be the major product?