- #1
Paintjunkie
- 50
- 0
- TL;DR Summary
- regarding the way the dipole moment operator is handled in perturbation theory. electric and magnetic cases
I am reading a PHD thesis online "A controlled quantum system of individual neutral atom" by Stefan Kuhr. In it on pg46, he has a Hamiltonian
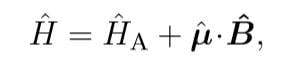
I am also reading a book by L. Allen "optical resonance and two level atoms" in it on page 34 he starts with a Hamiltonian where the perturbation is the same with the E operator instead of B.
They both develop this in the same manner saying that the dipole operator can be represented as
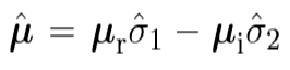
They both use the Heisenberg picture and eventually arrive at the following (Allen has E operator instead of B)
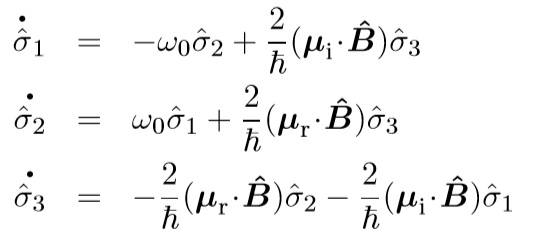
So I guess my question is. how come this is ok? Am I to assume the general treatment is fine, but when it comes down to actually calculating the dipole matrix elements they would be different? In my Jackson E&M book, on page 296, I see that E and B would have the same form, they are not really operators there though... I am just a little confused.
I myself as trying to use a Bloch Sphere to represent the interactions of resonant microwaves and an off resonant laser on a two level system. So I am thinking to develop it the same way the two of them do. I believe that it was Feynman, Vernon, and Hellwarth that developed this, but I have not actually looked at their derivation.
I am also reading a book by L. Allen "optical resonance and two level atoms" in it on page 34 he starts with a Hamiltonian where the perturbation is the same with the E operator instead of B.
They both develop this in the same manner saying that the dipole operator can be represented as
They both use the Heisenberg picture and eventually arrive at the following (Allen has E operator instead of B)
So I guess my question is. how come this is ok? Am I to assume the general treatment is fine, but when it comes down to actually calculating the dipole matrix elements they would be different? In my Jackson E&M book, on page 296, I see that E and B would have the same form, they are not really operators there though... I am just a little confused.
I myself as trying to use a Bloch Sphere to represent the interactions of resonant microwaves and an off resonant laser on a two level system. So I am thinking to develop it the same way the two of them do. I believe that it was Feynman, Vernon, and Hellwarth that developed this, but I have not actually looked at their derivation.
