Dario56
- 289
- 48
DLVO theory gives the curve of potential energy vs distance of two colloid particles. Potential energy curve is derived for colloids being only electrostatically stabilized and not sterically.
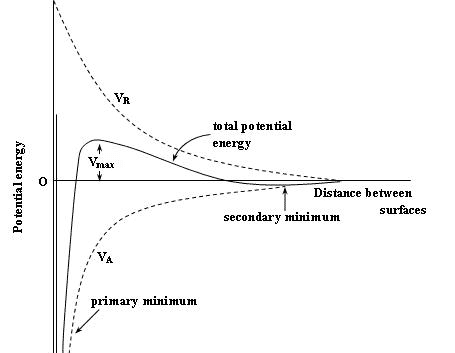
Looking at the image below which shows potential energy curve, we can see two local minima and one maximum. Between local maximum and secondary minimum is a domain of repulsive forces since potential energy increases by making distance between particles smaller.
Theory predicts that if colloid particles have enough energy (usually energy of Brownian motion), they can overcome repulsive forces and bond at primary local minimum which causes flocculation and colloid instability.
If they don't have enough energy colloid should be stable according to this theory, however this doesn't make sense since particles can still bond at secondary minimum. Bonding at secondary minimum forms much weaker bond which can be seen on the curve, but it should still cause particle bonding and flocculation since particles arrive at local minimum.
If this is so, how can this theory predict colloid stability?
Looking at the image below which shows potential energy curve, we can see two local minima and one maximum. Between local maximum and secondary minimum is a domain of repulsive forces since potential energy increases by making distance between particles smaller.
Theory predicts that if colloid particles have enough energy (usually energy of Brownian motion), they can overcome repulsive forces and bond at primary local minimum which causes flocculation and colloid instability.
If they don't have enough energy colloid should be stable according to this theory, however this doesn't make sense since particles can still bond at secondary minimum. Bonding at secondary minimum forms much weaker bond which can be seen on the curve, but it should still cause particle bonding and flocculation since particles arrive at local minimum.
If this is so, how can this theory predict colloid stability?