vcsharp2003
- 913
- 179
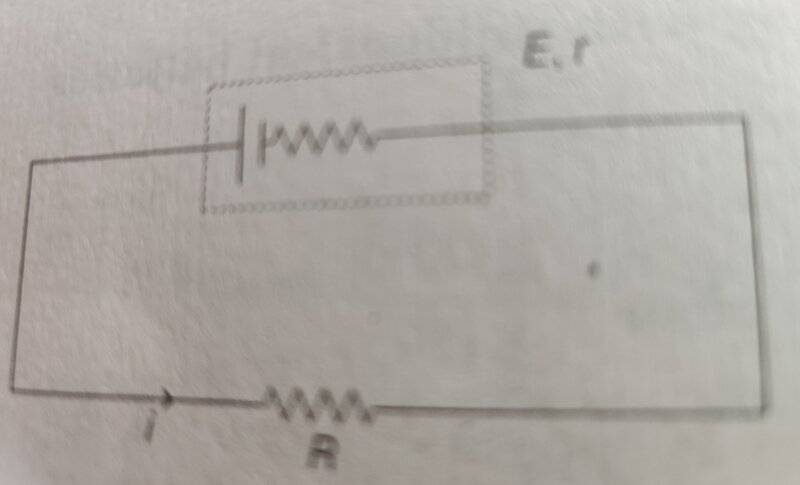
- Homework Statement
- In a closed circuit as shown in diagram below, a current is flowing i.e. electrons are flowing in a direction opposite to conventional current direction. What happens to the flowing electrons when they reach the positive terminal of the battery?
- Relevant Equations
- None
This is a confusing question. I am not sure if electrons flow through the battery from positive terminal to negative terminal against the electric field within the battery; or the electrons deposit on positive plate resulting in a chemical reaction that releases electrons at the negative plate which supplies free electrons to flow away from negative terminal in circuit.