Salmone
- 101
- 13
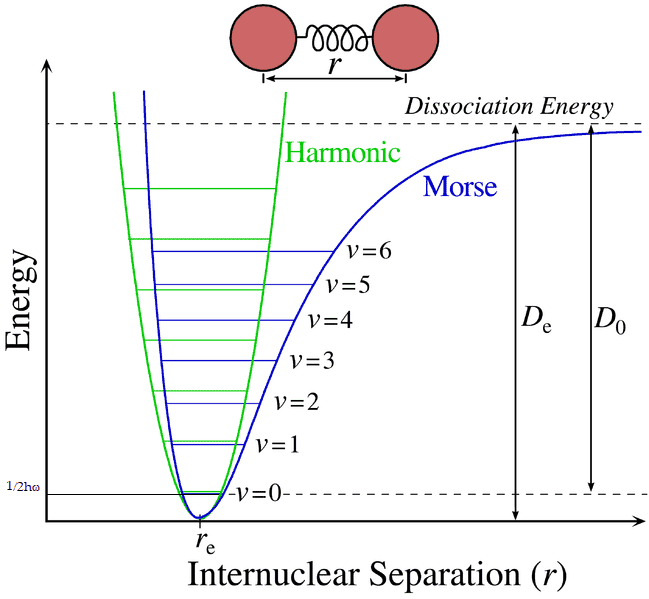
I have a little doubt about Morse potential used for vibration levels of diatomic molecules. With regard to the image below, if the diatomic molecule is in the vibrational ground state, when the oscillation reaches the maximum amplitude for that state the velocity of the molecule must be zero so that the kinetic energy will be zero and the Hamiltonian will be equal to the potential energy for that particular state. Now, since in quantum harmonic oscillator the eigenvalues of the Hamiltonian are equal to ##E=\hbar\omega(n+\frac{1}{2})##, for the ground state we have ##E=\frac{\hbar\omega}{2}##, so the total energy (kinetic + potential) must be always equal to that value in the G.S., for what I've wrote before then must be that the potential energy corresponding to the orizontal line of the vibrational ground state that is, the potential energy when the maximum amplitude is reached, is equal to ##E=\frac{\hbar\omega}{2}##, is it right?