LEO31
- 11
- 4
- Homework Statement
- Show the effects of different water temperatures on the dilution of 98% (w/w) H2SO4.
- Relevant Equations
- spreadsheet attached
I've been able to make a spreadsheet showing how the temperature changes depending on the final H2SO4 concentration in the diluite solution, but only if both the starting 98% acid and water are at ambient temperature. I'd like to figure out how to account for the difference in temperature of the starting acid (at different concentrations) and water.
For example: by starting with 98% (w/w) Acid at 323.15 °K and adding ambient temperature water
but also: by starting with 70% (w/w) Acid at 323.15 °K and adding water (@T≠ambient temperature)
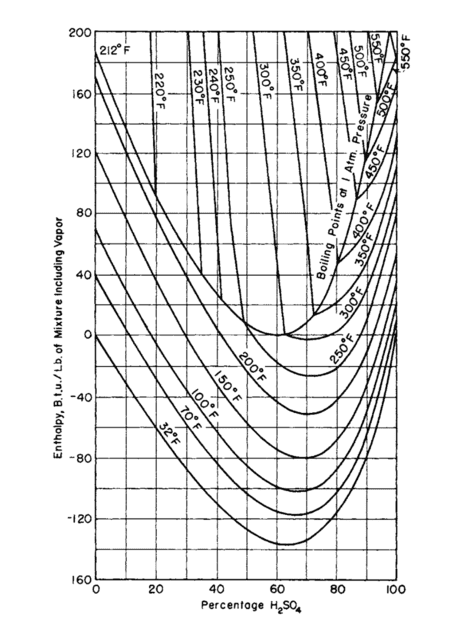
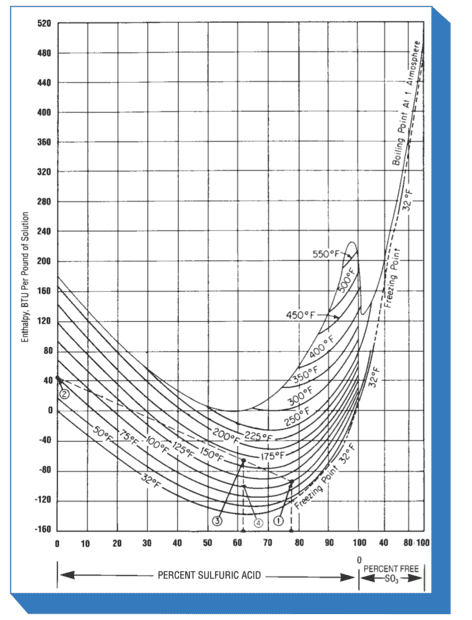
The only way I've found so far is to rely on some sort of graph such as the ones attached, which I know to be correct but I'd like know if there's a way to get a more accurate result without point guessing at a graph. Thanks !
For example: by starting with 98% (w/w) Acid at 323.15 °K and adding ambient temperature water
but also: by starting with 70% (w/w) Acid at 323.15 °K and adding water (@T≠ambient temperature)
The only way I've found so far is to rely on some sort of graph such as the ones attached, which I know to be correct but I'd like know if there's a way to get a more accurate result without point guessing at a graph. Thanks !