- #1
willib
- 227
- 0
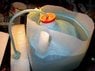
last night i tried to get hydrogen from water using the apparatus shown.
i have to improve my collection tecnique but i was able to collect about a third of a cup ful ..
say i collect it from the top of a dome shape device .
my question is how will i mix it with the oxygen , to burn it
i have to improve my collection tecnique but i was able to collect about a third of a cup ful ..
say i collect it from the top of a dome shape device .
my question is how will i mix it with the oxygen , to burn it