- #1
leopard
- 125
- 0
Why is a) more acidic than b) when C is more electron withdrawing than H? Electron withdrawal by a substituent increases acidity...
leopard said: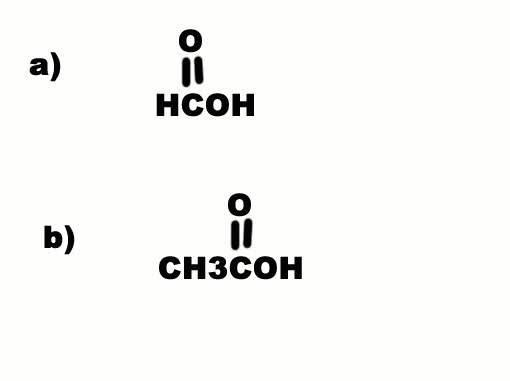
Why is a) more acidic than b) when C is more electron withdrawing than H? Electron withdrawal by a substituent increases acidity...
Electron withdrawal is the process by which an atom or molecule attracts electrons towards itself. This can occur through the presence of electronegative elements or functional groups. In terms of acidity, electron withdrawal can increase the stability of the conjugate base, making it more acidic.
Carbon and hydrogen are two of the most commonly found elements in organic molecules, which make up a large portion of our environment. Both of these elements can have varying levels of electronegativity, which can affect their ability to withdraw or donate electrons and thus impact the acidity of a molecule.
The relationship between carbon and hydrogen can affect acidity in several ways. The presence of electronegative elements or functional groups on the carbon atom can increase electron withdrawal, making the molecule more acidic. Additionally, the presence of hydrogen atoms can also impact the stability of the conjugate base and thus affect the acidity of a molecule.
While carbon and hydrogen generally follow the same trends in terms of their impact on acidity, there are exceptions. For example, in some cases, the presence of a hydrogen atom can actually increase the acidity of a molecule by stabilizing the conjugate base through intra-molecular bonding.
The relationship between carbon and hydrogen and acidity can be studied through various experimental and computational techniques. These include techniques such as NMR spectroscopy, X-ray crystallography, and computational modeling, which can provide insights into the electronic structure and acidity of a molecule. Additionally, the impact of different functional groups on the carbon atom can also be studied through chemical synthesis and analysis.