Apgomes3
- 7
- 2
Good morning,
I was wondering if I am thinking correctly. I am trying to establishing some step by step to calculate the amount of energy is being absorbed by a water body when a LED pendant is shining right above.
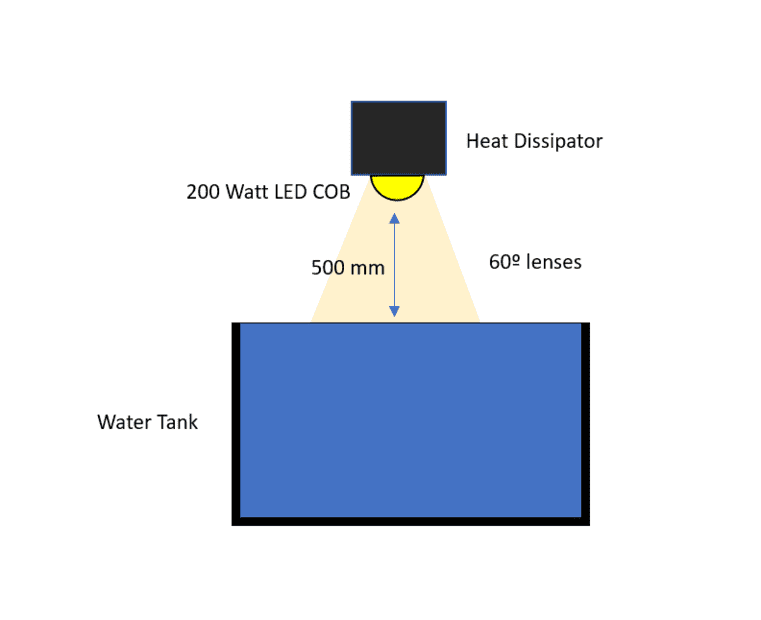
Being the parameter that I want to calculate, the energy absorbed by the water ( to calculate a proper heat exchanger to cool it down), what would you recommend as the best approach ?
I have the LED lux (I can get other parameters as well - as I have the light study for this specific light) more or less around 19103lx @ 1m
A bit lost in how to tackle properly.
Regards
I was wondering if I am thinking correctly. I am trying to establishing some step by step to calculate the amount of energy is being absorbed by a water body when a LED pendant is shining right above.
Being the parameter that I want to calculate, the energy absorbed by the water ( to calculate a proper heat exchanger to cool it down), what would you recommend as the best approach ?
I have the LED lux (I can get other parameters as well - as I have the light study for this specific light) more or less around 19103lx @ 1m
A bit lost in how to tackle properly.
Regards