VitorsSantiago
- 4
- 0
Hello everybody,
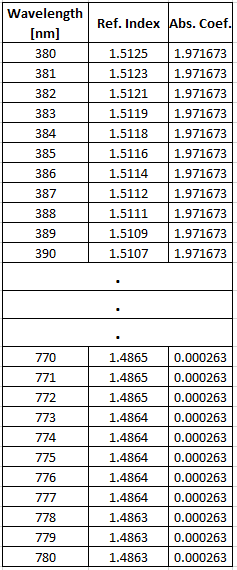
I would love some help on the following; I have a spectral date (As image 1 below) formed by the wavelength, refraction index and the absorption coefficient. Is there any way to relate this spectral date to a dominant wavelength? or in a few words extract the color from this spectrum?
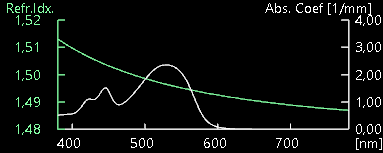
This information is also found in the spectrum file, I believe it is also usable in some way to calculate the dominant wavelength:

The real dominant color of this object is part of the red spectrum.
I would love some help on the following; I have a spectral date (As image 1 below) formed by the wavelength, refraction index and the absorption coefficient. Is there any way to relate this spectral date to a dominant wavelength? or in a few words extract the color from this spectrum?
This information is also found in the spectrum file, I believe it is also usable in some way to calculate the dominant wavelength:
The real dominant color of this object is part of the red spectrum.