- #1
Samson4
- 245
- 15
Another question and a another terrible illustration.
So my question: do faraday cages interfere with dispersion of ionic solutions?
In the illustration, a chemical reaction or some other cause for a lower concentration of ions is happening inside a faraday cage at B. Will the solution disperse into the cage as if it was not there?
A specific example: Let's say the solution is salt water. Inside the faraday cage, we are somehow removing ions from solution.
Will the ions outside the cage, move inside at a rate different than if there was no cage?
What if we only took positive ions from inside the cage? Now the area inside the cage is negatively charged. Will it stay that way for longer than it would without a cage present?
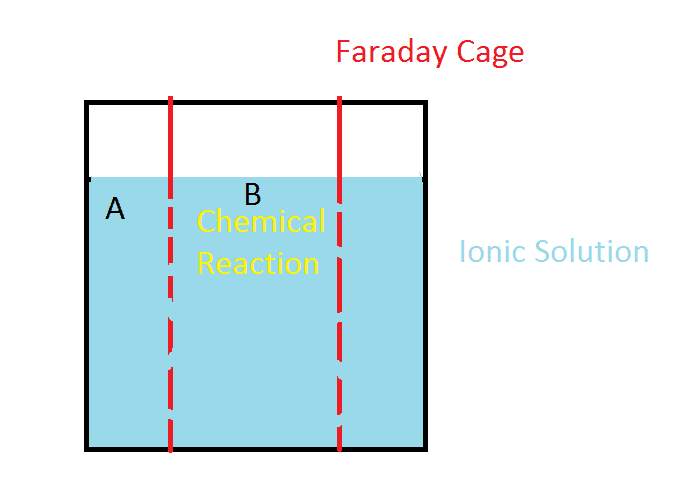
So my question: do faraday cages interfere with dispersion of ionic solutions?
In the illustration, a chemical reaction or some other cause for a lower concentration of ions is happening inside a faraday cage at B. Will the solution disperse into the cage as if it was not there?
A specific example: Let's say the solution is salt water. Inside the faraday cage, we are somehow removing ions from solution.
Will the ions outside the cage, move inside at a rate different than if there was no cage?
What if we only took positive ions from inside the cage? Now the area inside the cage is negatively charged. Will it stay that way for longer than it would without a cage present?