- #1
puggleboy
- 1
- 0
Homework Statement
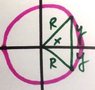
Problem 3 from the attached photo
Homework Equations
p = qd
The Attempt at a Solution
I know the magnitude of the dipole moment is p = qd, where d is the distance between the 2 oppositely charged particles. I know the direction of the dipole moment vector is from - to +. My first thought is there's no charge separation, and hence a dipole moment equals zero. Something tells me this isn't the case.