member 731016
- Homework Statement
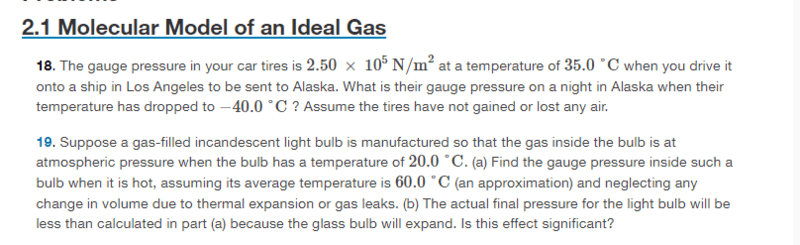
- Suppose a gas-filled incandescent light bulb is manufactured so that the gas inside the bulb is at atmospheric pressure when the bulb has a temperature of 20.0°C. (a) Find the gauge pressure inside such a bulb when it is hot, assuming its average temperature is 60.0°C (an approximation) and neglecting any change in volume due to thermal expansion or gas leaks. (b) The actual final pressure for the light bulb will be less than calculated in part (a) because the glass bulb will expand. Is this effect significant?
- Relevant Equations
- PV = nRT
For this 19(a),
The answer is 0.137 atm.
My working is
##P_{gauge} = P_f - P_i##
##P_{gauge} = \frac{nRT_f}{V_f} - \frac{nRT_i}{V_i}##
##P_{gauge} = \frac{nRT_f}{V} - \frac{nRT_i}{V}## since volume does not change
##P_{gauge} = \frac{nR}{V}(T_f - T_i)##
However, I am not sure how to go from here since we do not know the constant volume. Does someone please know how to tackle this?
Many thanks!
The answer is 0.137 atm.
My working is
##P_{gauge} = P_f - P_i##
##P_{gauge} = \frac{nRT_f}{V_f} - \frac{nRT_i}{V_i}##
##P_{gauge} = \frac{nRT_f}{V} - \frac{nRT_i}{V}## since volume does not change
##P_{gauge} = \frac{nR}{V}(T_f - T_i)##
However, I am not sure how to go from here since we do not know the constant volume. Does someone please know how to tackle this?
Many thanks!