- #1
Procrastinate
- 158
- 0
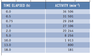
I am meant to deduce the half life from this table (as attached.) I was under the impression that the half life would occur when the initial activity was halved but my answer of 2.34 minutes (from halving the initial activity of 36506) is wrong. Instead, the answer the textbook got was 1 minute. What did I do wrong?