- #1
Asmaa Mohammad
- 182
- 7
Hello,
my book shows this figure of Fischer formula of D-glucose
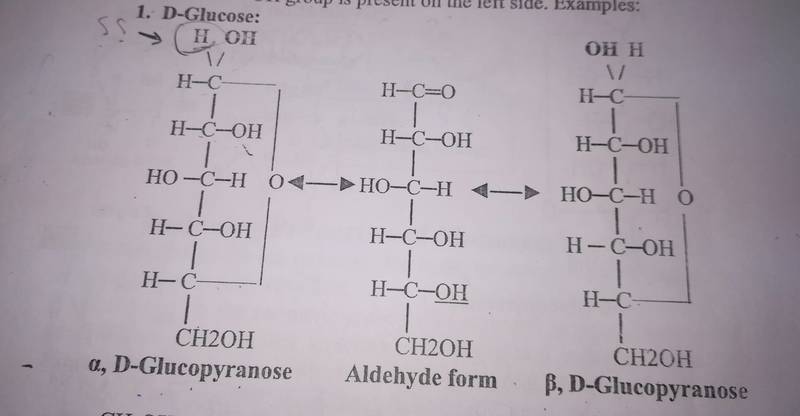
I don't understand this figure, and I wonder why the upper carbon atoms in both the right and left formulas have 5 bonds.
And from where comes this hedrogen atom in the upper left side of the figure (attached to alpha, D-glucopyranose).
When turning D-glucose to Fischer formula, it seems there's an additional hydrogen atom, isn't it? Why and how this happens?!
Could some one explain for me how the aldehyde form of glucose (the middle structure) to the beta, D-Glucopyranose and the alpha, D-Glucopyranose?
my book shows this figure of Fischer formula of D-glucose
I don't understand this figure, and I wonder why the upper carbon atoms in both the right and left formulas have 5 bonds.
And from where comes this hedrogen atom in the upper left side of the figure (attached to alpha, D-glucopyranose).
When turning D-glucose to Fischer formula, it seems there's an additional hydrogen atom, isn't it? Why and how this happens?!
Could some one explain for me how the aldehyde form of glucose (the middle structure) to the beta, D-Glucopyranose and the alpha, D-Glucopyranose?