- #1
Lucas Silva
- 4
- 0
New user has been reminded to fill out the Homework Help Template when starting a new schoolwork thread.
Suppose the hydrogen atom consists of a positive point charge (+e), located in the center of the atom, which is surrounded by a negative charge (-e), distributed in the space around it.
The space distribution of the negative charge changes according to the law p=Ce^(−2r/R), where C is a constant, r is the distance from the center of the atom, and R is Bohr's radius.
Find the value of the constant C by using the electrical neutrality of the atom.
Find the electrical field for r<R
Find the eltrical field for r > R
So what I've done is:
Since p stands for charge density, p = Q/V , where V = 4/3 Pi R^3 and Q = -Q
Thus, p = C*exp^(−2r/R) = -Q / 4/3 Pi R^3
Solving for C... C=[ -3*Q* r^2 * exp(2R/r)] / 4*Pi* R^3
For the electrical field I couldn't think of anything...
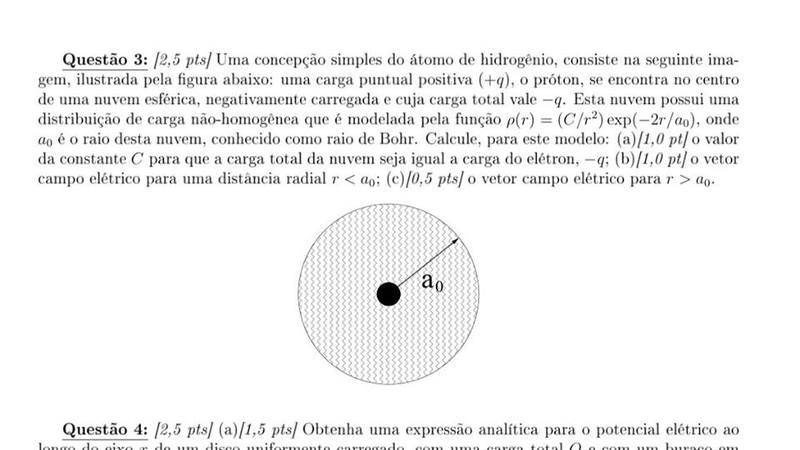
Sorry for the portuguese image... It was the only I could find. Note Ao = R
The space distribution of the negative charge changes according to the law p=Ce^(−2r/R), where C is a constant, r is the distance from the center of the atom, and R is Bohr's radius.
Find the value of the constant C by using the electrical neutrality of the atom.
Find the electrical field for r<R
Find the eltrical field for r > R
So what I've done is:
Since p stands for charge density, p = Q/V , where V = 4/3 Pi R^3 and Q = -Q
Thus, p = C*exp^(−2r/R) = -Q / 4/3 Pi R^3
Solving for C... C=[ -3*Q* r^2 * exp(2R/r)] / 4*Pi* R^3
For the electrical field I couldn't think of anything...
Sorry for the portuguese image... It was the only I could find. Note Ao = R


